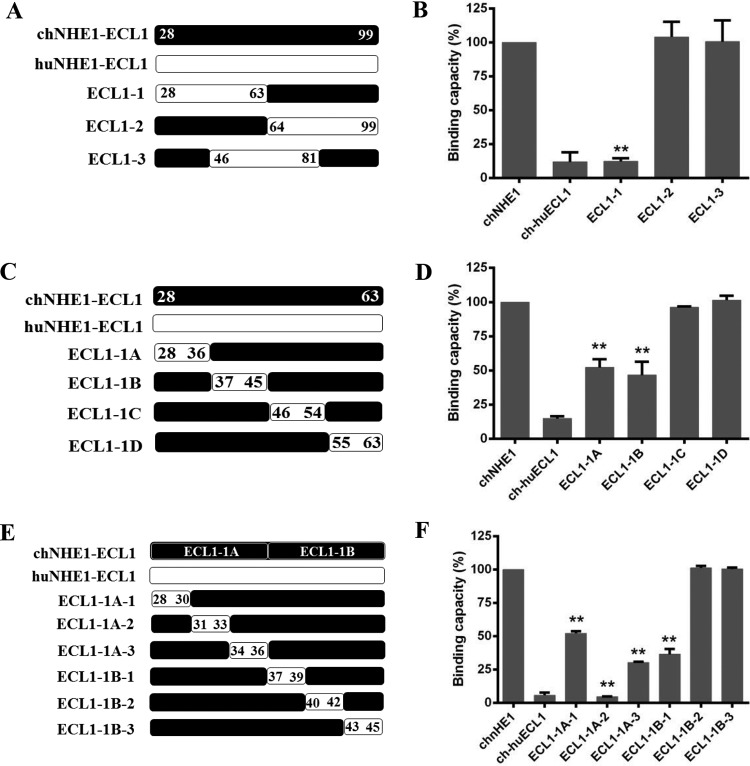
FIG 3.
Residues 28 to 39 in the N-terminal part of chECL1 constitute the minimal gp85-binding domain. (A) Schematic representation of the strategy for constructing chimeric chNHE1s with three equal-length chECL1 fragments replaced with the corresponding fragments of huECL1. (B) The gp85-binding capacity of 293T cells expressing ECL1-1, ECL1-2, and ECL1-3 chimeric receptors evaluated in a receptor binding assay. (C) Schematic representation of the strategy of constructing chimeric chNHE1s with four equal-length fragments of ECL1-1 replaced with the corresponding fragments of huECL1. (D) The relative gp85-binding capacity of 293T cells expressing ECL1-1A, ECL1-1B, ECL1-1C, and ECL1-1D chimeric receptors, evaluated in a receptor binding assay. (E) Schematic representation of the strategy of constructing chimeric chNHE1s with six equal-length segments of ECL1-1A and ECL1-1B replaced with the corresponding fragments of huECL1. (F) The relative gp85-binding capacity of 293T cells expressing ECL1-1A-1/2/3 and ECL1-1B-1/2/3 chimeric receptors, evaluated in a receptor binding assay. In panels A, C, and E, the black bars represent chECL1 and the white bars represent huECL1. (B, D, and F) Three independent experiments were performed, and data are shown as means ± standard deviations for triplicates from a representative experiment. **, P < 0.01.