FIG 4.
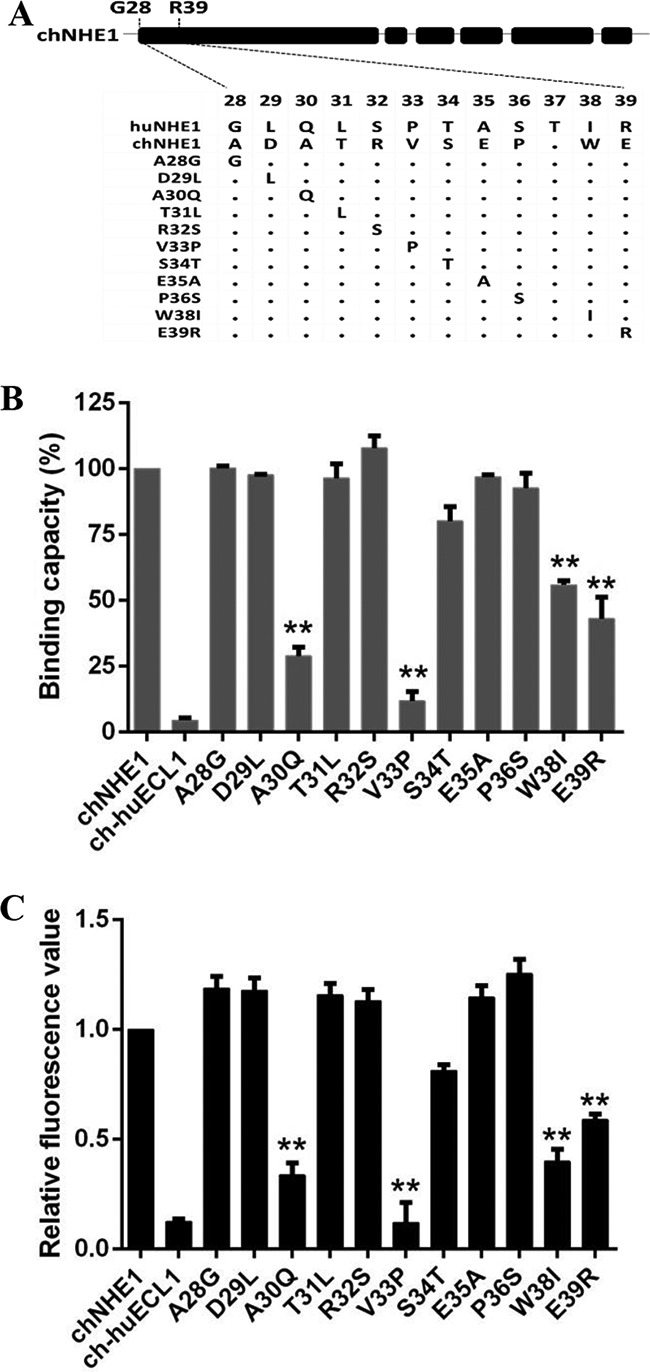
Residues A30, V33, W38, and E39 of ECL1 are key residues for the gp85-binding activity of chNHE1. Eleven amino acids from among residues 28 to 39 of chNHE1 were changed by site-directed mutagenesis to the corresponding amino acids of huNHE1, and the gp85-binding and viral entry-mediating activities of the chimeric receptors were evaluated. (A) Schematic representation of the strategy of constructing chimeric chNHE1s with 11 different single-residue substitutions. (B) The relative gp85-binding capacity of 293T cells expressing the wild type or different single-residue variants of chNHE1. The binding capacity of wild-type chNHE1 was set at 100%, and the values for the other receptors were calculated as proportions of the wild-type value. (C) Entry of RCAS(J)-luciferase virus into 293T cells expressing the wild type or different single-residue variants of chNHE1, assayed as described in the legend to Fig. 1. (B and C) Three independent experiments were performed, and data are shown as means ± standard deviations for triplicates from a representative experiment. **, P < 0.01.
