FIG 5.
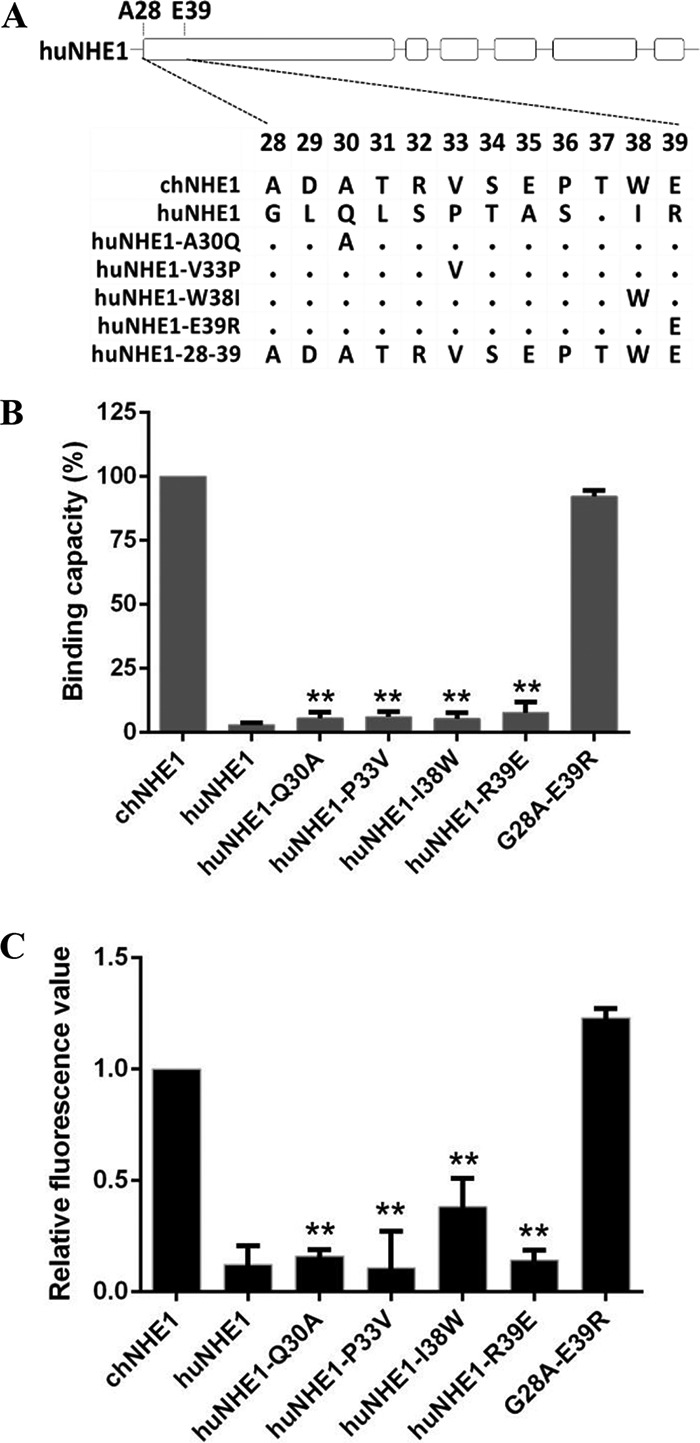
Residues 28 to 39 of chNHE1 are sufficient to convert huNHE1 into a functional ALV-J receptor and mediate virus entry. (A) Schematic representation of the strategy of constructing chimeric huNHE1s with single-residue or 12-amino-acid substitutions within ECL1. The huNHE1 residues were replaced with the corresponding chNHE1 residues. (B) The relative gp85-binding capacity of 293T cells expressing different chimeric huNHE1s. The binding capacity of wild-type chNHE1 was set at 100%, and the values for the chimeric huNHE1 receptors were calculated as its proportion. (C) Entry of RCAS(J)-luciferase virus into 293T cells expressing different chimeric huNHE1s, assayed as described in the legend to Fig. 1. (B and C) Three independent experiments were performed, and data are shown as means ± standard deviations for triplicates from a representative experiment. **, P < 0.01.
