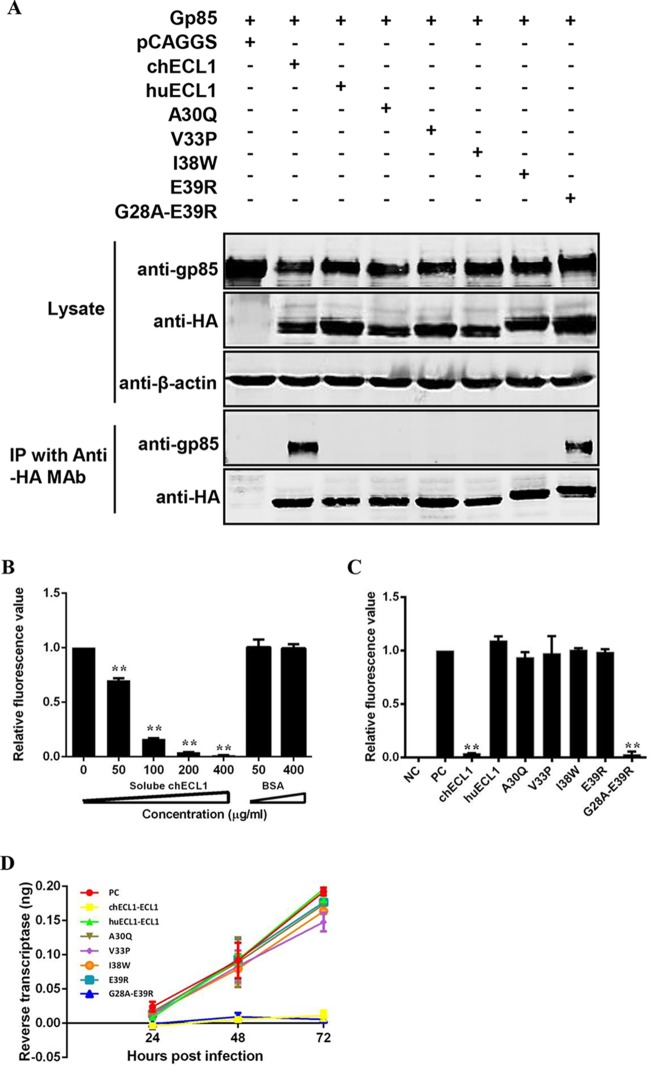
FIG 6.
Soluble huECL1 harboring the corresponding residues 28 to 39 of chNHE1 efficiently blocks ALV-J infection. (A) Coimmunoprecipitation (co-IP) validation of the interaction of gp85 and soluble chimeric huECL1s described in the legend to Fig. 5A. 293T cells were transfected with gp85- and the appropriate soluble chimeric huECL1-expressing plasmids. Cell lysates were prepared 48 h posttransfection, and the proteins were immunoprecipitated by anti-HA-agarose MAb; the proteins were immunoblotted with 4A3 anti-gp85 MAb or anti-HA antibodies. (B) Soluble chECL1 blocks RCAS(J)-luciferase virus entry into DF-1 cells. RCAS(J)-luciferase virus was incubated with different concentrations of soluble chECL1 or bovine serum albumin (BSA) for 1 h at 4°C. This was followed by infection of DF-1 cells. To detect viral entry, the cells infected with RCAS(J)-luciferase virus were lysed 24 h postinfection and luciferase activity was detected in a luciferase reporter assay. BSA was used as a negative control. (C and D) chECL1 and huECL1 harboring the corresponding residues 28 to 39 of chNHE1 block viral entry (C) and reduce the degree of viral replication (D). DF-1 cells were infected with RCAS(J)-luciferase virus or HPRS-103 preincubated with soluble chimeric huECL1s or chECL1. To detect viral entry, the cell culture infected with RCAS(J)-luciferase virus was lysed 24 h postinfection and the luciferase activity was determined by a luciferase reporter assay. To detect viral replication, the cell culture infected with HPRS-103 was harvested 24, 48, and 72 h postinfection, and RT activity was detected using an RT assay. Uninfected DF-1 cells were used as a negative control. RCAS(J)-luciferase virus or HPRS-103 that had not been preincubated with soluble chimeric receptors was used as a positive control. (B, C, and D) Three independent experiments were performed, and data are shown as means ± standard deviations for triplicates from a representative experiment. **, P < 0.01.