ABSTRACT
Innate immunity provides an immediate defense against infection after host cells sense danger signals from microbes. Endoplasmic reticulum (ER) stress arises from accumulation of misfolded/unfolded proteins when protein load overwhelms the ER folding capacity, which activates the unfolded protein response (UPR) to restore ER homeostasis. Here, we show that a mechanism for antiviral innate immunity is triggered after the ER stress pathway senses viral glycoproteins. When hemagglutinin (HA) glycoproteins from influenza A virus (IAV) are expressed in cells, ER stress is induced, resulting in rapid HA degradation via proteasomes. The ER-associated protein degradation (ERAD) pathway, an important UPR function for destruction of aberrant proteins, mediates HA degradation. Three class I α-mannosidases were identified to play a critical role in the degradation process, including EDEM1, EDEM2, and ERManI. HA degradation requires either ERManI enzymatic activity or EDEM1/EDEM2 enzymatic activity when ERManI is not expressed, indicating that demannosylation is a critical step for HA degradation. Silencing of EDEM1, EDEM2, and ERManI strongly increases HA expression and promotes IAV replication. Thus, the ER stress pathway senses influenza HA as “nonself” or misfolded protein and sorts HA to ERAD for degradation, resulting in inhibition of IAV replication.
IMPORTANCE Viral nucleic acids are recognized as important inducers of innate antiviral immune responses that are sensed by multiple classes of sensors, but other inducers and sensors of viral innate immunity need to be identified and characterized. Here, we used IAV to investigate how host innate immunity is activated. We found that IAV HA glycoproteins induce ER stress, resulting in HA degradation via ERAD and consequent inhibition of IAV replication. In addition, we have identified three class I α-mannosidases, EDEM1, EDEM2, and ERManI, which play a critical role in initiating HA degradation. Knockdown of these proteins substantially increases HA expression and IAV replication. The enzymatic activities and joint actions of these mannosidases are required for this antiviral activity. Our results suggest that viral glycoproteins induce a strong innate antiviral response through activating the ER stress pathway during viral infection.
KEYWORDS: innate immunity, UPR, influenza, EDEM1, EDEM2, ERManI, HA, NA, PAMP, PRR, EDEM3, ER stress, ERAD, hemagglutinin
INTRODUCTION
Innate immunity provides the most rapid host defense against microbial pathogen infection and also controls host adaptive immunity (1). Host pattern recognition receptors (PRRs) sense pathogen-associated molecular patterns (PAMPs) to elicit this immediate host defensive response, which releases type I interferons (IFNs) and proinflammatory cytokines/chemokines, resulting in an antimicrobial response. Currently, the best-characterized PAMPs in viral infection are viral nucleic acids, which are sensed by several classes of PRRs (2).
As obligate intracellular parasites, viruses hijack host endoplasmic reticulum (ER) to produce a large quantity of viral glycoproteins, resulting in ER stress (3). This stress arises from accumulation of misfolded/unfolded proteins in the ER when protein load overwhelms the ER folding capacity. ER stress is sensed by three ER transmembrane receptors, including the double-stranded RNA (dsRNA)-activated protein kinase-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) (4). These sensors activate a series of signaling cascades, known as the unfolded protein response (UPR), to reduce the aberrant proteins in the ER by increasing protein folding capacity, halting protein translation, and degrading misfolded proteins via the ER-associated protein degradation (ERAD) pathway. ERAD, an important mechanism by which UPR maintains ER homeostasis, retrotransports misfolded proteins from the ER to the cytoplasm for degradation via the ubiquitin/proteasome system (5). Class I α-mannosidases, which specifically cleave the four α1,2-linked mannose residues distributed on the three sugar branches A, B, and C of N-glycan, play an important role in ERAD (6). These enzymes consist of seven members, including the ER class I α-mannosidase (ERManI), ER degradation-enhancing α-mannosidase-like (EDEM) proteins 1, 2, and 3, and Golgi class I mannosidases (GolgiMan) IA, IB, and IC. If glycoproteins are folded properly in the ER, only the outermost mannose residue on branch B is cleaved, which allows native proteins to enter the Golgi complex and complete N-glycosylation. However, if glycoproteins are terminally misfolded, the remaining three α1,2-mannose residues are cleaved in the ER, triggering misfolded protein degradation via ERAD (7).
Influenza A viruses (IAVs) are enveloped negative-sense RNA viruses that cause severe respiratory illness (8). Hemagglutinin (HA) and neuraminidase (NA) are two major viral glycoproteins on the viral surface that divide IAVs into a number of subtypes (9). HA is a primary determinant for IAV pathogenesis and is subject to N-glycosylation via 5 to 14 Asn-X-Ser/Thr motifs in the ER (10). The HA precursor HA0 proteins are processed into surface HA1 subunits that contain a receptor-binding domain and transmembrane HA2 subunits that contain a fusion peptide. HA1 and HA2 are linked by a disulfide bond and assemble as homotrimers to mediate IAV entry. Here, we report that HA is sensed by the ER stress pathway, which triggers a robust innate anti-IAV response via ERAD.
RESULTS
Induction of ER stress by IAV infection.
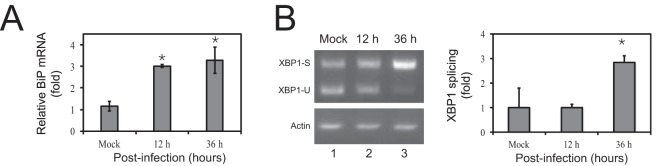
To determine whether IAV infection induces ER stress, we analyzed expression of the binding immunoglobulin protein (BiP), which is a master UPR regulator that is induced upon ER stress (11). A549 cells were infected with H1N1 A/WSN/33 virus at a multiplicity of infection (MOI) of 1, and BiP expression was measured by real-time quantitative PCR (qPCR) at 0, 12, and 36 h postinfection. BiP mRNAs were increased ∼3-fold by the infection (Fig. 1A), indicating an induction of UPR by IAV. To determine the downstream signaling pathway, we analyzed the IRE1 pathway in these infected cells, which reportedly was activated during IAV infection (12, 13). IRE1 is an ER transmembrane protein that has both endonuclease and Ser/Thr kinase activities. IRE1 is activated upon binding to unfolded proteins, which in turn activates a transcription factor X-box binding protein 1 (XBP1) by unconventionally splicing a 26-nucleotide intron out of the XBP1 mRNA. XBP1 then translocates to the nucleus and upregulates ER chaperones to promote ER folding capacity and ERAD components to increase misfolded protein degradation (14). We directly measured XBP1 mRNA splicing by PCR at 0, 12, and 36 h postinfection. The spliced form of XBP1 mRNA was increased by 2-fold at 36 h postinfection (Fig. 1B). These results demonstrate that UPR is induced and IRE1 is activated upon IAV infection.
FIG 1.
Induction of ER stress by IAV infection. (A) BiP expression was analyzed in H1N1 A/WSN/33 virus-infected A549 cells by real-time qPCR at the indicated time points postinfection. (B) XBP1 splicing was analyzed in the same H1N1 A/WSN/33 virus-infected A549 cells by PCR. The spliced (S) XBP1, unspliced (U) XBP1, and actin bands are indicated. Error bars represent standard deviations (SD) from three independent experiments. y axes represent normalized fold changes, where mock infection is considered 1-fold.
Activation of the IRE1 pathway by HA.
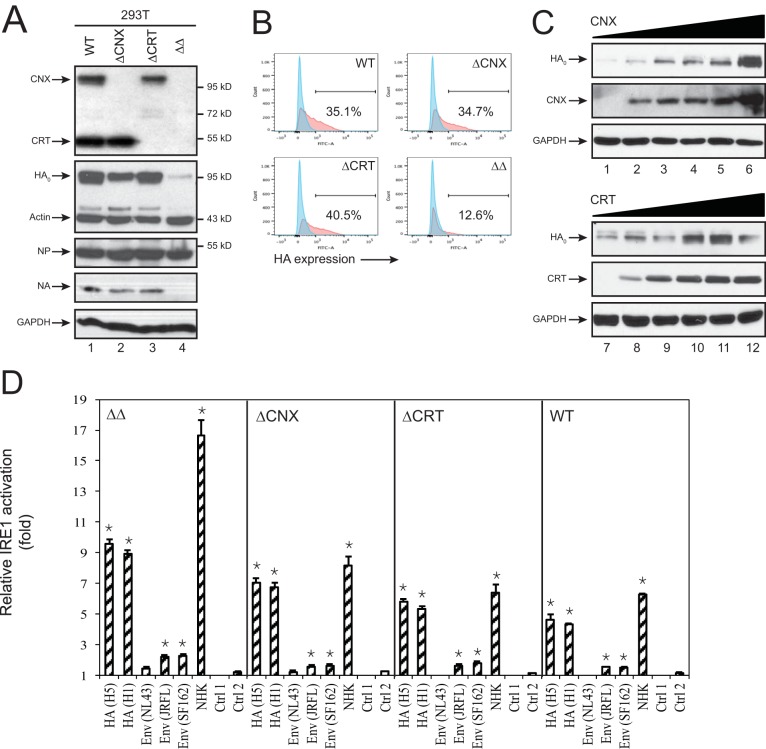
HA is one of the major IAV glycoproteins that is productively expressed in the ER after infection. To understand how HA expression affects ER homeostasis, we studied the regulation of HA biosynthesis. Calnexin (CNX) and calreticulin (CRT) comprise a fundamental chaperone system in the ER that promotes protein folding and N-glycosylation (15) and were reported to interact with IAV HA (16–18). Previously, we created CNX- and/or CRT-knockout (KO) cell lines using CRISPR/Cas9 (19). We examined HA subtype 5 (H5) expression in these cells. H5 has 9 N-glycosylation sites. When H5 precursor expression was analyzed by Western blotting, there was a pronounced decrease in HA0 expression in double-KO (ΔΔ) cells compared to wild-type (WT) and single-KO cells (Fig. 2A, lane 4). This decrease in steady-state expression translated into lower HA1 expression on the surface of the ΔΔ cells when analyzed by flow cytometry (Fig. 2B). We also examined expression of IAV NA from subtype 1 (N1) that has 4 N-glycosylation sites as well as the viral nucleoprotein (NP) in these cells. NA is reported to interact with CNX and CRT (20), while the nonglycosylated NP does not depend on the secretory pathway. Consistent with this, N1, but not NP, expression was inhibited in ΔΔ cells (Fig. 2A). To further confirm that the HA expression defect in ΔΔ cells was caused by the absence of CNX/CRT and was not an off-target artifact, CNX/CRT were reconstituted via ectopic expression. HA expression was restored through ectopic reconstitution of CNX or CRT in a dose-dependent manner (Fig. 2C, lanes 1 to 6 and lanes 7 to 12). Thus, the functional requirement for CNX/CRT in IAV HA and NA expression are demonstrated in these experiments.
FIG 2.
Activation of the IRE1 pathway by HA. (A) Wild-type (WT), ΔCNX, ΔCRT, and ΔΔ 293T cell lines were transfected with an IAV HA (H5), NA (N1), or NP expression vector, and protein expression was analyzed via Western blotting using the indicated antibodies. (B) HA cell surface expression in WT, ΔCNX, ΔCRT, and ΔΔ 293T cells was determined by flow cytometry. (C) HA expression in ΔΔ cells was rescued by ectopic expression of CNX or CRT in a dose-dependent manner. (D) The XBP1 splicing was determined after transient transfection of the pXBP1u-FLuc reporter and viral glycoprotein expression vectors for HIV-1 and IAV into WT, ΔCNX, ΔCRT, and ΔΔ 293T cells. HA proteins from IAV subtypes H1 and H5 and Env proteins from HIV-1 NL43, JRFL, and SF162 were used. NHK, terminally misfolded human alpha1-antitrypsin variant null (Hong Kong). Control 1 (Ctrl 1) was from untransfected cells, and Ctrl 2 was from cells only transfected with the pXBP1u-FLuc vector. Results are displayed as the means ± standard errors of the means (SEM) (n = 3). *, P < 0.05 by unpaired two-tailed t test. The y axis represents normalized fold changes, where Ctrl 1 is considered 1-fold.
To determine whether ER stress is elicited and UPR is activated, we chose to use a luciferase-based reporter assay to measure IRE1-mediated XBP1 activation (21). As a positive control for UPR induction, the terminally misfolded human alpha1-antitrypsin variant null (Hong Kong) (NHK) was used (22). IAV HAs from H1 and H5 subtypes, the human immunodeficiency virus type 1 (HIV-1) envelope (Env) glycoproteins from the NL43, JRFL, and SF162 strains, and NHK were ectopically expressed with the XBP1 reporter construct in WT, ΔCNX, ΔCRT, and ΔΔ 293T cells, and levels of IRE1 activation were analyzed. HIV-1 Env expression resulted in marginal IRE1 activation, whereas the activation was significantly increased by IAV HA expression in all these cells (Fig. 2D). In addition, much higher levels of IRE1 activation by HA were observed in KO cells than in WT cells (Fig. 2D). These results demonstrate that HA alone is able to activate the IRE1 pathway, which is consistent with the previous report (13).
HA degradation via ERAD.
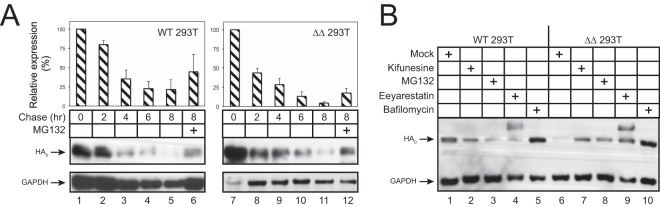
To determine how HA expression is suppressed, we analyzed HA protein stability in ΔΔ cells. WT and ΔΔ 293T cells were transfected with an H5 expression vector and treated with cycloheximide (CHX), and protein levels were assessed after 2, 4, 6, and 8 h. HA half-life in WT cells was ∼4 h, which was reduced to less than 2 h in ΔΔ cells (Fig. 3A). These results suggest that HA is rapidly turned over in ΔΔ cells. To understand how HA is degraded, cells were treated with the proteasome inhibitor MG132 or the lysosome inhibitor bafilomycin A1. Bafilomycin A1 increased HA expression in both WT and ΔΔ cells (Fig. 3B, lanes 5 and 10), whereas MG132 selectively increased the HA expression in ΔΔ cells (Fig. 3B, lanes 3 and 8). In addition, MG132 increased the HA half-life more significantly in ΔΔ than in WT cells in the stability analysis (Fig. 3A, lanes 6 and 12). These results demonstrate that HA is predominately degraded via proteasomes, indicating that HA is not folded properly in ΔΔ cells.
FIG 3.
HA degradation via ERAD. (A) WT and ΔΔ 293T cells were transfected with an H5 expression vector, and the HA steady state was chased at the indicated time points by Western blotting after treatment with cycloheximide (CHX) at 50 μg/ml. The relative HA expression was measured by quantification of the intensity of each protein band on the blot using ImageJ. Error bars represent SD from three independent experiments. y axes represent percent changes, where the value at time zero is considered 100%. (B) After transfection of WT or ΔΔ 293T cells with an H5 expression vector, cells were incubated with MG132 (25 μM), kifunensine (5 μM), eeyarestatin (50 μM), or bafilomycin A1 (100 nM) for 4 h and analyzed by Western blotting.
To test the role of ERAD in HA degradation, we treated cells with two other compounds that block ERAD at different steps. ERAD involves three major steps, including substrate recognition, retrotranslocation into the cytosol, and degradation via the ubiquitin proteasome system. Class I α-mannosidases promote the recognition step by catalyzing extensive demannosylation, which can be blocked by their specific enzymatic inhibitor, kifunesine (6). p97 AAA-ATPases are required for ERAD substrate retrotranslocation from the ER lumen to the cytosol by catalyzing ATP hydrolysis, which is blocked by eeyarestatin I (23). Both kifunesine and eeyarestatin I blocked HA degradation in ΔΔ cells as effectively as MG132 (Fig. 3B, lanes 7 and 9). These results demonstrate that HA is indeed rapidly degraded via ERAD in ΔΔ cells.
Inhibition of HA expression by EDEM1, EDEM2, and ERManI.
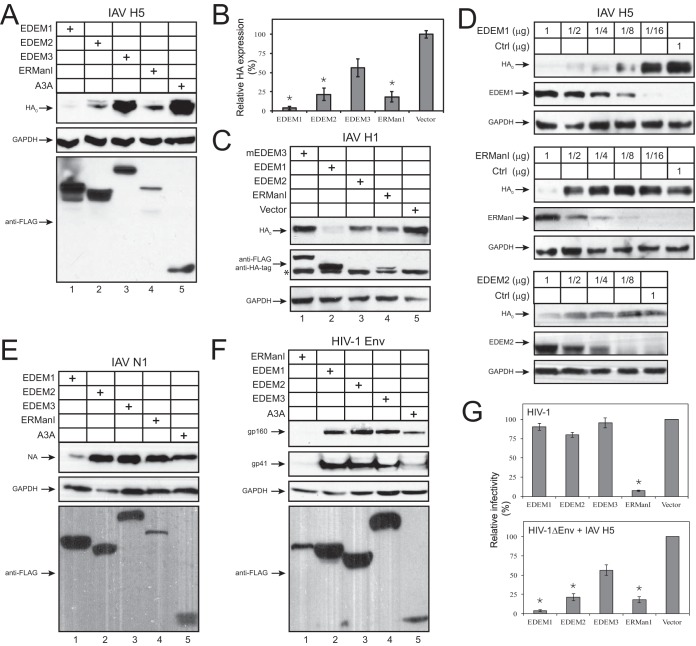
Having discovered HA degradation via ERAD in ΔΔ cells, we further studied the degradation mechanism in WT 293T cells. Since kifunesine rescues HA expression, we tested whether HA could be targeted by the four mannosidases in the ER, including ERManI, EDEM1, EDEM2, and EDEM3. We expressed H5 with these class I α-mannosidases, and protein expression levels were determined by Western blotting. Ectopic expression of EDEM1, EDEM2, and ERManI resulted in strong 4- to 10-fold inhibition of HA expression, with EDEM1 showing the strongest effect, while EDEM3 showed a marginal effect (Fig. 4A and B). To confirm the low activity of EDEM3, we repeated this experiment by expressing murine EDEM3 (mEDEM3) and IAV HA of another subtype, H1, that contains 6 N-glycosylation sites. Again, EDEM1, EDEM2, and ERManI showed similar inhibitory activities, and EDEM3 did not show any effect on H1 expression (Fig. 4C). To determine the specificity of EDEM1, EDEM2, and ERManI, their dose-dependent effects were determined by varying their protein expression levels. Inhibition of HA expression correlated with the levels of EDEM1, EDEM2, and ERManI expression, supporting their specific inhibitory effects (Fig. 4D).
FIG 4.
Inhibition of HA expression by EDEM1, EDEM2, and ERManI. (A) 293T cells were transfected with an H5 expression vector and a vector expressing EDEM1, EDEM2, or ERManI with a C-terminal FLAG tag. After 48 h, cells were lysed and analyzed via Western blotting. APOBEC3A (A3A) was used as a control. (B) HA expression on Western blots shown in panel A was quantified with ImageJ and is presented as relative values. Results are displayed as the means ± SD (n = 3). *, P < 0.05 by unpaired two-tailed t test. (C) A similar experiment was conducted by replacing H5 with H1 and human EDEM3 with murine EDEM3 (mEDEM3). An unspecific band, which overlaps the EDEM2 band, is labeled with an asterisk. (D) 293T cells were transfected with a fixed amount of H5 and increasing amounts of EDEM1, EDEM2, or ERManI expression vector (in micrograms), and HA expression was analyzed via Western blotting. (E and F) The effect of EDEM and ERManI on IAV N1 expression (E) and that of EDEM and ERManI on HIV-1 Env expression (F) were similarly determined. (G) Effect of EDEM and ERManI on single-cycle viral replication. HIV-1 with authentic Env or HIV-1 pseudoviruses with IAV H5 and N1 were produced by transfection of an EDEM or ERManI expression vector plus pNL4-3 or pNL-GFP proviral vector, respectively. The infectivity of natural HIV-1 was determined after infection of HIV-1 luciferase reporter cell line TZM-b1 cells and measurement of the intracellular luciferase activity; the infectivity of HA-pseudotyped HIV-1 was determined after infection of MDCK cells and measurement of the intracellular green fluorescent protein (GFP) expression. Results are displayed as the means ± SEM (n = 3). *, P < 0.05 by unpaired two-tailed t test. y axes in panels B and G represent percent changes, where the value from the vector control is considered 100%.
Because NA expression also was suppressed in ΔΔ cells, we tested whether NA could be targeted by these mannosidases. When N1, which has 4 N-glycosylation sites, was expressed with EDEM and ERManI proteins, we found that EDEM1 also inhibited the NA expression but EDEM2, EDEM3, and ERManI did not (Fig. 4E). As a comparison, we determined how EDEM and ERManI proteins inhibit expression of HIV-1 Env, which contains ∼26 N-glycosylation sites. Similar to what we previously reported (24), only ERManI inhibited Env expression (Fig. 4F, lane 1).
We next used a single-cycle viral replication assay to confirm these results by generating HA-pseudotyped HIV-1 infectious particles. HIV-1 pseudovirions were produced from 293T cells after cotransfection of the Env-deficient HIV-1 proviral vector pNLΔEnv with IAV H5, IAV N1, and EDEM or ERManI expression vectors. After normalization of virus production by HIV-1 p24Gag enzyme-linked immunosorbent assay (ELISA), viral infectivity was determined. The infectivity of these HIV-1 pseudoviruses was strongly inhibited by ERManI, EDEM1, and EDEM2 but not EDEM3, with EDEM1 showing the strongest activity; the infectivity of natural HIV-1 with authentic Env was inhibited only by ERManI (Fig. 4G). These results demonstrate that EDEM1, EDEM2, and ERManI specifically inhibit IAV HA expression, suggesting that these class I mannosidases play an important role in the intracellular degradation process. In addition, the lower levels of ERManI expression suggest a higher specific activity on IAV HA substrate than do EDEM1 and EDEM2.
Requirement of ERManI, EDEM1, and EDEM2 catalytic activity for HA degradation.
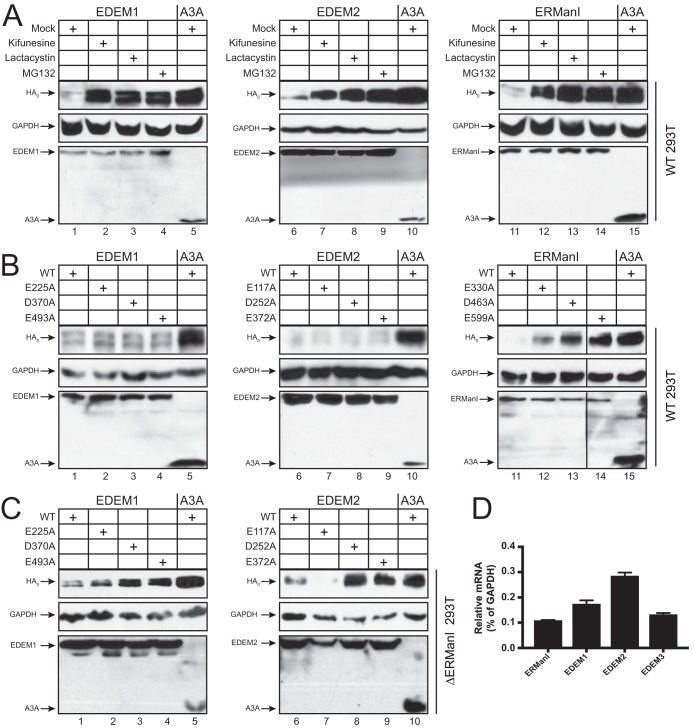
Previously, we reported that class I α-mannosidase activity is required for ERManI inhibition of HIV-1 Env expression (24). In contrast, this catalytic activity is not required for ERManI inhibition of misfolded human NHK protein expression (25). To better understand the EDEM and ERManI inhibitory mechanism, we tested whether catalytic activity is required for inhibition of HA expression. We compared their inhibitory effects in the presence of kinfunesine as well as the proteasome inhibitors lactacystin and MG132. Kifunesine blocked HA degradation by ERManI, EDEM1, and EDEM2 as effectively as lactacystin and MG132 (Fig. 5A). Thus, ERManI, EDEM1, and EDEM2 catalytic activities are required for HA degradation.
FIG 5.
Requirement of mannosidase activity for HA inhibition. (A) 293T cells were transfected with an H5 vector and the indicated EDEM and ERManI expression vectors. After 24 h, cells were treated with kifunensine (5 μM), lactacystin (25 μM), or MG132 (25 μM) for 6 h, and protein expression was analyzed by Western blotting. (B) 293T cells were transfected with an H5 vector and the indicated EDEM and ERManI WT or catalytic mutant expression vector, and protein expression was analyzed by Western blotting. (C) ERManI-KO 293T cells were transfected with an H5 vector and the indicated EDEM WT or mutant expression vector, and protein expression was analyzed by Western blotting. (D) The relative expression of ERManI, EDEM1, EDEM2, and EDEM3 in the human lung epithelial cell line A549 was determined by real-time qPCR. Results are displayed as means ± SEM (n = 3). *, P < 0.05 by unpaired two-tailed t test. The y axis represents relative mRNA levels (%) after normalization to the levels of GAPDH.
We next mutated the putative catalytic residues in these proteins and tested whether their activities could be disrupted. Although EDEM class I α-mannosidase activities have not been demonstrated in vitro, the three ERManI catalytic residues (E330, D463, and E599) are well conserved in EDEM1 (E225, D370, and E493) and EDEM2 (E117, D252, and E372) (26). Previously, we created three ERManI catalytic mutants, E330A, D463A, and E599A (24). Analogous EDEM1 catalytic mutants E225A, D370A, and E493A and EDEM2 catalytic mutants E117A, D252A, and E372A were created. When these mutants were compared to their WT proteins, all EDEM1 and EDEM2 catalytic mutants still were able to inhibit HA expression, whereas the ERManI mutants lost inhibitory activity against HA expression (Fig. 5B). These results suggest that ERManI requires its catalytic activity to inhibit HA expression but EDEM1 and EDEM2 do not.
To reconcile these conflicting results, we tested these EDEM1 and EDEM2 catalytic mutants again in a previously reported ERManI-KO cell line (24). When HA was expressed with these mutants, the two EDEM1 mutants D370A and E493A and two EDEM2 mutants D252A and E373A lost their inhibitory activity, although the EDEM1 E225A and EDEM2 E117A mutants were still as active as their WT proteins (Fig. 5C), indicating the requirement for their catalytic activity in this cell line. This result suggests that the catalytic activity of EDEM1 and EDEM2 becomes indispensable for inhibition of HA expression when ERManI is not present. ERManI, EDEM1, EDEM2, and EDEM3 expression all were detectable in the human lung epithelial cell line A549 by qPCR, indicating that all four of these mannosidases are expressed in these cells (Fig. 5D). Thus, ERManI, EDEM1, and EDEM2 likely all play a role in targeting HA to ERAD for degradation.
Inhibition of HA expression by endogenous EDEM1, EDEM2, and ERManI.
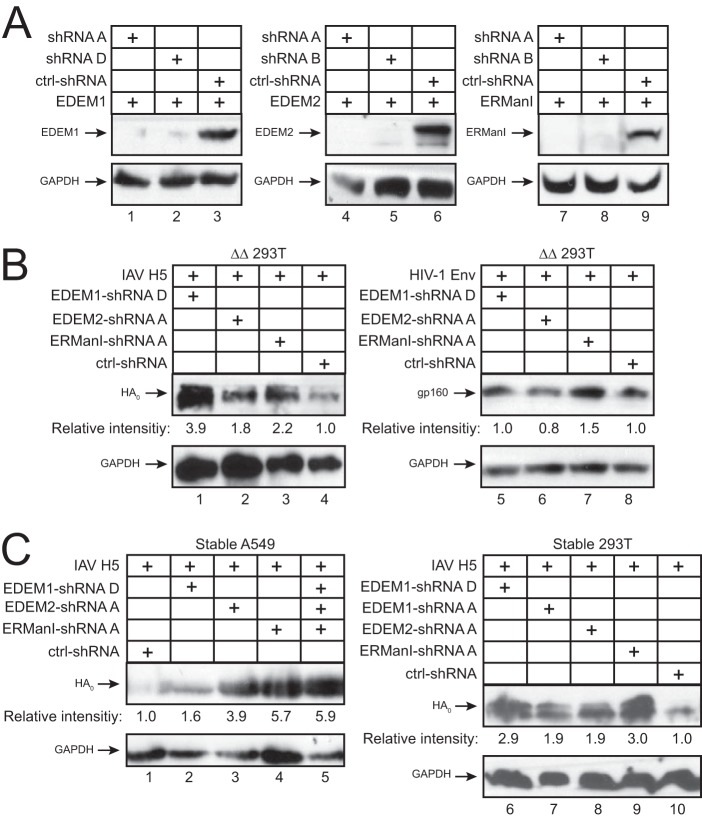
To further validate the inhibitory effects of ERManI, EDEM1, and EDEM2, we used short hairpin RNAs (shRNAs) to silence their expression. Two sets of shRNAs specific to each enzyme were expressed from a lentiviral vector, and their gene knockdown (KD) efficiencies were determined by Western blotting. Compared to a scrambled shRNA control (ctrl-shRNA), these specific shRNAs effectively reduced EDEM1, EDEM2, or ERManI expression, respectively (Fig. 6A, lanes 1 to 9).
FIG 6.
Inhibition of HA expression by endogenous EDEM1, EDEM2, and ERManI. (A) WT 293T cells were transfected with EDEM1, EDEM2, and ERManI expression vectors plus a lentiviral vector expressing the specific shRNAs or a scrambled shRNA as a control (ctrl). Protein expression was analyzed by Western blotting. (B) ΔΔ 293T cells were transfected with an H5 or HIV-1 proviral vector plus a lentiviral vector expressing the indicated shRNAs. Protein expression was analyzed by Western blotting. (C) WT A549 and 293T cells were stably transduced with a lentiviral vector expressing the indicated shRNAs, and HA expression in these cells was analyzed by Western blotting.
After confirming their knockdown efficiencies, we tested whether these specific shRNAs increase IAV HA and HIV-1 Env expression in the ΔΔ cells. When these shRNAs were expressed with HA or Env, all of them increased HA expression, with the EDEM1 shRNAs having the most potent effect (Fig. 6B, lanes 1 to 3). HIV-1 Env expression was slightly increased by the ERManI shRNAs (Fig. 6B, lane 7). These results are consistent with our previous finding that HA is targeted by EDEM1, EDEM2, and ERManI, whereas HIV-1 Env is only targeted by ERManI.
We next created A549 cell lines stably expressing these shRNAs via lentiviral transduction. When HA was expressed in these A549 cells, its expression was effectively increased by the EDEM2 and ERManI shRNAs and less effectively by the EDEM1 shRNAs (Fig. 6C, lanes 2 to 4). We also created a triple-KD A549 cell line silencing all three genes. When HA expression was tested in these triple-KD cells, a much stronger increase in HA expression was observed (Fig. 6C, lane 5). To understand why the EDEM1 shRNAs did not show a stronger effect, these shRNAs were also stably expressed in 293T cells via lentiviral transduction. When HA expression was tested, the EDEM1 shRNAs exhibited an efficiency in enhancing expression similar to that of the EDEM2 and ERManI shRNAs (Fig. 6C, lanes 6 to 9). These results further confirm the specific inhibition of HA expression by EDEM1, EDEM2, and ERManI.
Inhibition of IAV replication by EDEM1, EDEM2, and ERManI.
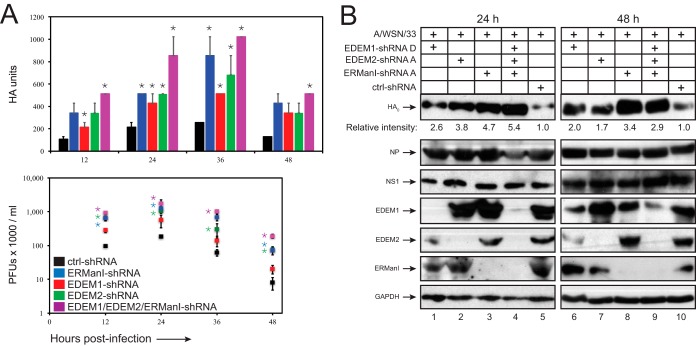
After establishment of the stable A549 silencing cell lines, we infected these cells with A/WSN/33 IAV (an H1N1 IAV in which the HA has 6 and the NA has 4 N-glycosylation sites) at an MOI of 0.5 (27). Viral supernatants were collected at 12, 24, 36, and 48 h postinfection, and viral replication was determined by measuring hemagglutination titers using turkey blood cells or by determining the PFU after infecting MDCK cells. Single KDs increased hemagglutination titers maximally by 2- to 4-fold, whereas the triple KD increased the titers by 5-fold at 36 h postinfection (Fig. 7A). Notably, more prominent effects were detected from the plaque assay. During the entire infection, the triple KD exhibited the strongest effect by increasing PFU 10- to 24-fold; single KDs also increased PFU 3- to 7-fold, and the ERManI-KD had the strongest effect (Fig. 7A; note the log scale). The triple KD does not appear significantly different from the ERManI KD. However, the difference is more evident at 48 h. Like the hemagglutination titers, the number of PFU dropped significantly at 48 h, which is likely caused by the cytopathic effect from viral infection. To confirm that enhancement of viral replication is linked to increased HA expression via the shRNAs, we determined expression of EDEM1, EDEM2, and ERManI as well as viral proteins HA, NP, and nonstructural protein 1 (NS1) in these infected cells by Western blotting at 24 and 48 h postinfection. The expression of EDEM1, EDEM2, and ERManI was effectively reduced during the infection by their respective shRNAs (Fig. 7B). In addition, only the expression of HA, but not NP or NS1, was significantly increased by these shRNAs. Importantly, the triple KD and ERManI KD increased the HA expression more strongly than the other two single KDs. Taken together, these results identify EDEM1, EDEM2, and ERManI as critical host factors that are able to inhibit IAV replication by blocking the expression of HA and possibly NA as well.
FIG 7.
Inhibition of IAV replication by EDEM1, EDEM2, and ERManI. (A) Stable A549 cell lines expressing the indicated shRNAs were infected with H1N1 A/WSN/33 viruses at an MOI of 0.5. Viral supernatants were sampled at the specified time points. Viral titers were determined by a hemagglutination assay using turkey red blood cells and plaque-forming cell assay after infecting MDCK cells. Results are displayed as means ± SD (n = 2). *, P < 0.05 by unpaired two-tailed t test. (B) Infected cells at 24 h and 48 h from the same infection experiments were collected and analyzed by Western blotting using the indicated antibodies. The relative intensity of HA0 was measured using ImageJ software.
DISCUSSION
The ER is responsible for not only translation but also proper folding of secreted proteins, which account for one-third of total cellular proteins. To secrete native and fully functional proteins, the ER is equipped with multiple chaperone systems and folding enzymes that promote protein maturation. Nevertheless, protein folding is complex and susceptible to errors, resulting in accumulation of misfolded/unfolded proteins that induce ER stress (28). Accordingly, eukaryotic cells have evolved UPR as an evolutionarily conserved stress response mechanism to maintain the ER homeostasis. Production of viral proteins places an added burden on the folding machinery, and ER homeostasis is easily disrupted by productive viral replication. Consequently, UPR is frequently induced in virally infected cells to support cellular function and, at the same time, benefit chronic viral infection (3). Notably, UPR signaling cascades have been found to intersect with inflammatory and interferon pathways, so it is speculated that the UPR should have an additional function for sensing viral infection as part of the innate immune antiviral response (29). Recently, Hrincius et al. reported that HA is sensed by an ER stress pathway, resulting in inflammatory responses that cause acute lung damage in IAV-infected mice (13). Here, we present evidence that UPR sensing of HA triggers a direct anti-IAV response by targeting HA degradation via ERAD. Collectively, our findings strongly suggest that host cells detect HA as a misfolded or “nonself” protein, as proposed by Hrincius et al., and that ER mannosidases target HA to ERAD for degradation.
ERAD is a host quality control mechanism that specifically targets misfolded glycoproteins for degradation, ensuring that only properly folded proteins are secreted from the ER. As obligate intracellular parasites, viruses have been found to exploit ERAD to promote viral replication (30–32). Viruses may degrade host proteins such as major histocompatibility complex class I and CD4 via ERAD to escape the host immune response or avoid superinfection, resulting in enhanced viral replication. In addition, viruses may produce viral proteins from, enter through, or replicate in some components of the ERAD machinery to directly promote viral replication. Here, we demonstrate that ERAD can be recruited to degrade HA expression and inhibit IAV replication.
We have identified three class I α-mannosidases, ERManI, EDEM1, and EDEM2, that trigger HA degradation and inhibit IAV replication. These three enzymes are responsible for HA degradation not only in CNX/CRT KO cells but also in WT cells. Class I α-mannosidases are responsible for cleavage of the four α1,2-linked mannose residues distributed on the three N-glycan branches A, B, and C. Currently, it is not clear how extensive demannosylation is accomplished in the ER to initiate ERAD. ERManI cleaves the outermost mannose residue on branch B, whereas the three Golgi mannosidases cleave the other three α1,2-linked mannose residues in vitro. However, ERManI also cleaves these three mannose residues at high concentrations (33). Thus, in the course of viral infection, where viral proteins may be greatly overexpressed, ERManI may play a very central role, cleaving residues on branches A, B, and C. This is consistent with our finding that ERManI is the most active mannosidase in HA degradation and IAV inhibition. While our data strongly indicate that class I α-mannosidase enzymatic activity is required for HA degradation, inactivation of the catalytic residues selectively disrupted the ERManI, but not the EDEM1 and EDEM2, inhibition of HA expression in WT 293T cells. This again is consistent with a central role for ERManI in directing HA to ERAD. Furthermore, inactivation of EDEM1 and EDEM2 activity only shows a phenotype in ERManI-KO cells. Collectively, our data point toward ERManI playing the key role in trimming the α1,2-Man residues and directing HA to ERAD. The fact that silencing any one of EDEM1, EDEM2, and ERManI results in an increase of HA expression and IAV replication does, however, present a conundrum. Perhaps this can be reconciled by a noncatalytic role for EDEM1 and EDEM2, where the interaction of HA with these mannosidases facilitates the cleavage of mannose groups by ERMan1. We found all three mannosidases expressed in human lung epithelial cells, a natural target for IAV infection, so it is plausible that these proteins can collaborate in some fashion to initiate the process of HA degradation. Thus, ERManI, EDEM1, and EDEM2 are important players in this innate antiviral response in the ER to inhibit IAV replication.
MATERIALS AND METHODS
Chemicals and antibodies.
The anti-FLAG antibody, kifunensine, l-(tosylamido-2-phenyl) ethyl chloromethyl ketone (TPCK)-treated trypsin, and cycloheximide were purchased from Sigma. Bafilomycin A1, MG132, and lactacystin were purchased from Santa Cruz. Eeyarestatin was purchased from Calbiochem. Concentrations of the inhibitors used are as stated here unless otherwise noted: cycloheximide (50 μg/ml), kifunensine (5 μM), eeyarestatin (50 μM), bafilomycin A1 (100 nM), lactacystin (25 μM), and MG132 (25 μM). Mouse monoclonal anti-ERManI (3C2) was purchased from Novus Biologicals; rabbit polyclonal anti-EDEM1 and anti-EDEM2 were purchased from Sigma; a goat anti-actin polyclonal antibody was purchased from Santa Cruz Biotechnology; a mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was purchased from Meridian Life Science. Horseradish peroxidase (HRP)-conjugated human, rabbit, goat, horse, and mouse immunoglobulin G secondary antibodies were purchased from Pierce. HIV-1 proteins were detected by antibodies from the NIH AIDS Research and Reference Reagent Program, and their catalogue numbers are 526 (HIV-1 gp41) and 521 (HIV-1 gp120). Mouse anti-HA2, anti-NA, anti-NS1, and anti-NP were purchased from BEI Resources (NR-44222, NR-13459, NR-44426, and NR-4282, respectively).
Cell lines.
Human embryonic kidney cells 293 carrying the simian virus 40 T antigen (293T), human lung carcinoma cell line A549, and Madin-Darby canine kidney (MDCK) epithelial cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% bovine calf serum (BCS), which was purchased from HyClone. ERMan1-KO 293T cells were described previously (24). 293T CNX/CRT single- and double-KO cells were described previously (19). Stable 293T and A549 cell lines expressing ERManI, EDEM1, and EDEM2 shRNAs were generated through lentiviral transduction. Lentiviral particles were produced through cotransfection of the Origene pGFP-C-shLenti vectors (catalog numbers TL313302A, TL313302D, TL304849A, and TL303362A), the packaging vector pCMVΔ8.9, and vesicular stomatitis virus glycoprotein expression vector. These viruses were used to infect 293T or A549 cells and cultured in the presence of puromycin (10 μg/ml). The infection was also confirmed with fluorescence microscopy to test green fluorescent protein (GFP) expression. Stable knockdown cell lines were further confirmed by the expression of GFP, which was expressed from the same lentiviral vector.
Plasmids.
pCMV6 entry vectors expressing human EDEM1, EDEM2, and EDEM3; pcDNA3.1 expression vectors for human APOBEC3A (A3A), ERManI, and ERManI mutants E330A, D463A, and E599A; and the murine EDEM3-HA expression vector were described previously (24). Plasmids expressing IAV [A/Thailand/1(KAN-1)/2004(H5N1) strain] HA (7705) and NA (7708) were provided by Gary Nabel. pHW181-PB2, pHW182-PB1, pHW183-PA, pHW184-HA, pHW185-NP, pHW186-NA, pHW187-M, and pHW-188-NS were provided by Robert G. Webster (27). pCMVΔ8.9 was provided by Didier Trono. pXBP1u-FLuc, for measuring XBP1 splicing, was provided by Yi-Ling Lin (21). The HIV-1 proviral vectors pNL4-3 and pNL4-3.Luc.R−E− were obtained from the NIH AIDS Research and Reference Reagent Program. pNL-Luc was constructed by swapping the BamH1-XhoI fragment from pNL4-3.Luc.R−E−. pNL-GFP was constructed by replacing the firefly luciferase gene with the enhanced GFP gene after NotI and XhoI digestion. pcDNA-EDEM1-FLAG-HA and pcDNA-EDEM2-FLAG-HA were constructed by replacing the A3G gene in the pcDNA3.1-A3G-HA-FLAG vector that expresses an in-frame C-terminal tandem-arrayed HA-FLAG tag after HindIII and NotI digestion. Using the QuikChange II site-directed mutagenesis kit (Agilent), EDEM1 E225A, D370A, and E493A mutants were created in the pCMV6-Entry-EDEM1 vector, and EDEM2 E117A, D252A, and E372 mutants were created in the pcDNA-EDEM2-HA-FLAG vector. pGFP-C-shLenti lentiviral vectors expressing 4 unique 29mer shRNAs (A, B, C, and D) against human ERManI (TL302262), EDEM1 (TL313302), EDEM2 (TL304849), and a scrambled control (TR30021) were purchased from OriGene.
Real-time qPCR analysis.
Total RNAs were extracted from mock- or virus-infected A549 cells using an RNA extraction kit (Qiagen, Chatsworth, CA). First-strand complementary DNAs (cDNAs) were synthesized using ReverTra Ace qPCR RT master mix (Toyobo, Japan) according to the manufacturer's instructions. Real-time PCRs were performed using SYBR Premix Ex Taq II (TaKaRa, China) in a LightCycler 480 II real-time PCR system (Roche). Primers for EDEM1 are 5′-CAGAATAATAACTGACTCCAAGCAGC-3′ and 5′-CTGTCTTTAGATTCACCCGAGGAT-3′; primers for EDEM2 are 5′-ATTCCAAAGAGTGGTTGAAGTGC-3′ and 5′-CAGCCTTCTTGGAGAGCAGATGA-3′; primers for ERManI are 5′-CCAGCAAATCCACCCGTCTTAC-3′ and 5′-GGTCTTGGCTTGGGGGTCTAAT-3′; primers for BiP are 5′-AGGCTTATTTGGGAAAGAAGGTTAC-3′ and 5′-GATCCTCATAACATTTAGGCCAGC-3′; and primers for GAPDH are 5′-GAGTCAACGGATTTGGTCGT-3′ and 5′-GGTGCCATGGAATTTGCCAT-3′. The thermal cycling conditions were the following: 1 cycle of 95°C for 5 min, followed by 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 20 s. Fluorescence signal was acquired continuously after the last elongation step to monitor dissociation. Melting curves were obtained with MxPro software. Expression levels of the target genes were computationally transformed from the threshold cycle (CT) value and then normalized by GAPDH expression. Results were determined by the 2−ΔΔCT method.
XBP1 mRNA splicing assay.
The assay was performed as described previously, with minor modifications (34). Briefly, A549 cells were infected with H1N1 WSN/33 viruses at an MOI of 0.5. Total RNAs were extracted from the infected cells at 0, 12, and 24 h postinfection. After reverse transcription, 2 μl of cDNAs were used as templates for amplification of the XBP1 mRNAs with the described primers (34), using 60°C as the annealing temperature. The PCR products were analyzed on a 2% agarose gel after PstI digestion.
Transfection.
Polyethylenimine (PEI) stock solution (1 mg/ml) was prepared by dissolving 500 mg PEI (Polysciences) into 500 ml sterilized water after adjusting the pH to 4.5 with HCl and filtering the solution through a 0.22-μm membrane. To produce HA (7705)-pseudotyped HIV-1 and express various mannosidase proteins, 1 × 106 293T cells were seeded in each well of a 6-well culture plate 16 h before transfection. Three micrograms of total DNA was diluted into 200 μl serum-free DMEM and mixed with 9 μg PEI. After 15 min of incubation at room temperature, these transfection reagents were added directly into the supernatant of each well. Media were replaced after 6 h and cell lysate collected at 48 h unless otherwise noted. Viruses were collected from the supernatants, and viral production was measured by p24Gag ELISA; protein expression was directly determined by Western blotting.
Analysis of HA-pseudotyped HIV-1 infectivity.
Viral particles were produced from 293T cells cotransfected with pNL-GFP, HA expression vector 7705, and NA expression vector 7708. Particles were normalized by p24Gag ELISA, and equal amounts of viruses were used to infect MDCK cells. After 48 h, cells were washed 3 times with PBS, filtered through 40-μm nylon mesh, and used for flow cytometry analysis.
Analysis of HIV-1 infectivity.
HIV-1 particles were produced from 293T cells after transfection with pNL4-3 and a mannosidase expression vector or vector control. After normalization by p24Gag ELISA, equal amounts of viruses were used to infect TZM-b1 cells. After 48 h, cells were lysed and viral infectivity was determined by measuring the cellular luciferase activity using the firefly luciferase reporter assay kit from Promega.
Production of H1N1 A/WSN/33 virus and hemagglutination assay.
A/WSN/33 virus was produced as described before using an 8-plasmid transfection into MDCK/HEK 293T coculture (27). Viral titer was determined via MDCK plaque assay. For infection experiments, 0.5 × 106 A549 stable knockdown cells were seeded into a 6-well plate and incubated for 6 h. Media then were replaced with Opti-MEM supplemented with 0.5 μg/ml TPCK trypsin, and virus was added at an MOI of 0.5. Viral supernatants were collected at 12-, 24-, 36-, and 48-h time points, and extracted media were replaced with fresh Opti-MEM (0.5 μg/ml TPCK trypsin). Hemagglutination titers were determined by a turkey blood HA assay using 2-fold dilutions of the virus. HA units were calculated from the last dilution of virus that exhibited turkey red blood cell agglutination. Viral titers, in PFU per milliliter, were calculated by plaque assay using 12-well plates. Adsorption of 10-fold serial dilutions of virus was performed on MDCK monolayers and overlaid with a 0.3% final concentration of agarose in Opti-MEM with 2 μg/ml TPCK trypsin. After 48 h, cells were fixed with 2% formaldehyde and stained with 0.15% crystal violet in ethanol for plaques to be detected.
Statistics.
Statistical tests were performed using Microsoft Excel. Significance of differences between samples was assessed using an unpaired two-tailed Student's t test. Variance was estimated by calculating the standard deviations (SD) or the standard errors of the means (SEM) in each group, as indicated in figure legends, and are represented by error bars. Unless specified in the legend, all experiments were performed independently at least three times, and n indicates the number of biological replicates, with a representative experiment being shown.
ACKNOWLEDGMENTS
We thank Ian York and Richard N. Sifers for constructive comments on the manuscript. We thank Robert G. Webster, Yi-Ling Lin, Richard N. Sifers, Chengjun Li, BEI Resources, and the NIH AIDS Research and Reference Reagent Program for providing various reagents.
D.A.F. is supported by the Bertina Wentworth endowed fellowship and Russell B. Duvall endowed fellowship. B.W. is supported by grants from the National Natural Science Foundation (31502089), Natural Science Foundation of Heilongjiang Province (QC2017028), and Harbin Veterinary Research Institute (SKLVBP2017012), China. Y.H.Z. is supported by a grant from the “948” plan, 2014-Z10, Ministry of Agriculture, China.
REFERENCES
- 1.Iwasaki A, Medzhitov R. 2015. Control of adaptive immunity by the innate immune system. Nat Immunol 16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Z, Damania B. 2016. The cGAS-STING defense pathway and its counteraction by viruses. Cell Host Microbe 19:150–158. doi: 10.1016/j.chom.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan SW. 2014. The unfolded protein response in virus infections. Front Microbiol 5:518. doi: 10.3389/fmicb.2014.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Kaufman RJ. 2014. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer 14:581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 5.Vembar SS, Brodsky JL. 2008. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol 9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mast SW, Moremen KW. 2006. Family 47 alpha-mannosidases in N-glycan processing. Glycobiology 415:31–46. doi: 10.1016/S0076-6879(06)15003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hebert DN, Molinari M. 2012. Flagging and docking: dual roles for N-glycans in protein quality control and cellular proteostasis. Trends Biochem Sci 37:404–410. doi: 10.1016/j.tibs.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed). 2013. Fields virology, 6th ed Lippincott Williams & Wilkins Health, Philadelphia, PA. [Google Scholar]
- 9.Gamblin SJ, Skehel JJ. 2010. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J Biol Chem 285:28403–28409. doi: 10.1074/jbc.R110.129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tate MD, Job ER, Deng YM, Gunalan V, Maurer-Stroh S, Reading PC. 2014. Playing hide and seek: how glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection. Viruses 6:1294–1316. doi: 10.3390/v6031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo S, Mao C, Lee B, Lee AS. 2006. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol 26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan IH, Zhang MS, Powers LS, Shao JQ, Baltrusaitis J, Rutkowski DT, Legge K, Monick MM. 2012. Influenza A viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1) stress pathway. J Biol Chem 287:4679–4689. doi: 10.1074/jbc.M111.284695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hrincius ER, Liedmann S, Finkelstein D, Vogel P, Gansebom S, Samarasinghe AE, You D, Cormier SA, McCullers JA. 2015. Acute lung injury results from innate sensing of viruses by an ER stress pathway. Cell Rep 11:1591–1603. doi: 10.1016/j.celrep.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. 2013. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol 5:a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caramelo JJ, Parodi AJ. 2008. Getting in and out from calnexin/calreticulin cycles. J Biol Chem 283:10221–10225. doi: 10.1074/jbc.R700048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond C, Braakman I, Helenius A. 1994. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci U S A 91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebert DN, Foellmer B, Helenius A. 1996. Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes. EMBO J 15:2961–2968. [PMC free article] [PubMed] [Google Scholar]
- 18.Hebert DN, Zhang JX, Chen W, Foellmer B, Helenius A. 1997. The number and location of glycans on influenza hemagglutinin determine folding and association with calnexin and calreticulin. J Cell Biol 139:613–623. doi: 10.1083/jcb.139.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B, Wang Y, Frabutt DA, Zhang X, Yao X, Hu D, Zhang Z, Liu C, Zheng S, Xiang SH, Zheng YH. 2017. Mechanistic understanding of N-glycosylation in Ebola virus glycoprotein maturation and function. J Biol Chem 292:5860–5870. doi: 10.1074/jbc.M116.768168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang N, Glidden EJ, Murphy SR, Pearse BR, Hebert DN. 2008. The cotranslational maturation program for the type II membrane glycoprotein influenza neuraminidase. J Biol Chem 283:33826–33837. doi: 10.1074/jbc.M806897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu CY, Hsu YW, Liao CL, Lin YL. 2006. Flavivirus infection activates the XBP1 pathway of the unfolded protein response to cope with endoplasmic reticulum stress. J Virol 80:11868–11880. doi: 10.1128/JVI.00879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Swulius MT, Moremen KW, Sifers RN. 2003. Elucidation of the molecular logic by which misfolded alpha 1-antitrypsin is preferentially selected for degradation. Proc Natl Acad Sci U S A 100:8229–8234. doi: 10.1073/pnas.1430537100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garza RM, Sato BK, Hampton RY. 2009. In vitro analysis of Hrd1p-mediated retrotranslocation of its multispanning membrane substrate 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase. J Biol Chem 284:14710–14722. doi: 10.1074/jbc.M809607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou T, Frabutt DA, Moremen KW, Zheng YH. 2015. ERManI (endoplasmic reticulum class I alpha-mannosidase) is required for HIV-1 envelope glycoprotein degradation via endoplasmic reticulum-associated protein degradation pathway. J Biol Chem 290:22184–22192. doi: 10.1074/jbc.M115.675207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iannotti MJ, Figard L, Sokac AM, Sifers RN. 2014. A Golgi-localized mannosidase (MAN1B1) plays a non-enzymatic gatekeeper role in protein biosynthetic quality control. J Biol Chem 289:11844–11858. doi: 10.1074/jbc.M114.552091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivari S, Molinari M. 2007. Glycoprotein folding and the role of EDEM1, EDEM2 and EDEM3 in degradation of folding-defective glycoproteins. FEBS Lett 581:3658–3664. doi: 10.1016/j.febslet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valastyan JS, Lindquist S. 2014. Mechanisms of protein-folding diseases at a glance. Dis Model Mech 7:9–14. doi: 10.1242/dmm.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JA. 2014. A new paradigm: innate immune sensing of viruses via the unfolded protein response. Front Microbiol 5:222. doi: 10.3389/fmicb.2014.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byun H, Gou Y, Zook A, Lozano MM, Dudley JP. 2014. ERAD and how viruses exploit it. Front Microbiol 5:330. doi: 10.3389/fmicb.2014.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frabutt DA, Zheng YH. 2016. Arms race between enveloped viruses and the host ERAD machinery. Viruses 8:255. doi: 10.3390/v8090255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noack J, Bernasconi R, Molinari M. 2014. How viruses hijack the ERAD tuning machinery. J Virol 88:10272–10275. doi: 10.1128/JVI.00801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herscovics A, Romero PA, Tremblay LO. 2002. The specificity of the yeast and human class I ER alpha 1,2-mannosidases involved in ER quality control is not as strict previously reported. Glycobiology 12:14G–15G. [PubMed] [Google Scholar]
- 34.Saeed M, Suzuki R, Watanabe N, Masaki T, Tomonaga M, Muhammad A, Kato T, Matsuura Y, Watanabe H, Wakita T, Suzuki T. 2011. Role of the endoplasmic reticulum-associated degradation (ERAD) pathway in degradation of hepatitis C virus envelope proteins and production of virus particles. J Biol Chem 286:37264–37273. doi: 10.1074/jbc.M111.259085. [DOI] [PMC free article] [PubMed] [Google Scholar]