Figure 1. Tsc2 is a new client of Hsp90.
-
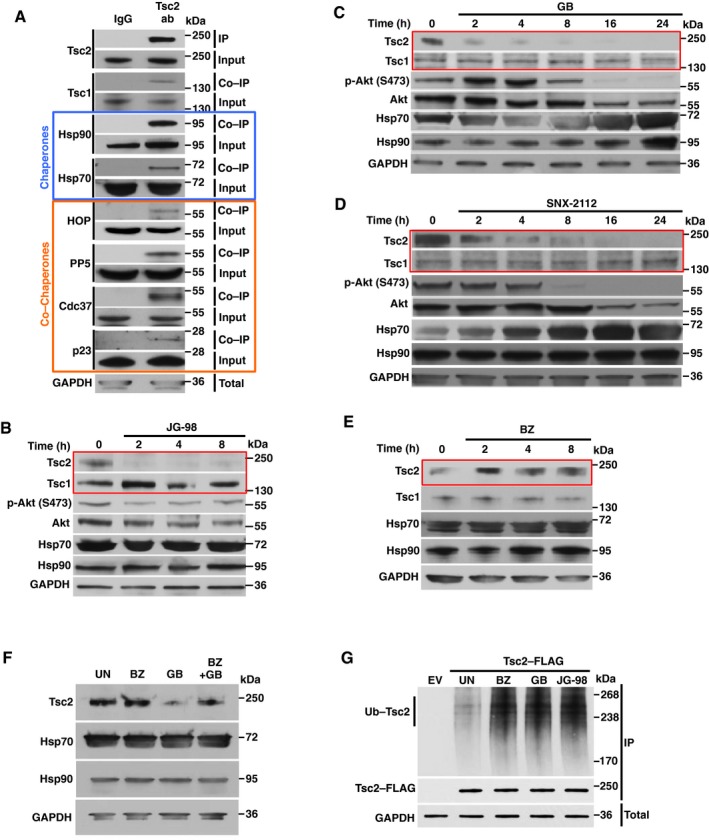
ATsc2 was immunoprecipitated from HEK293 cell lysates using anti‐Tsc2 antibody or IgG (control) and immunoblotted with indicated antibodies to confirm chaperone and co‐chaperone interaction.
-
BHEK293 cells were treated with 10 μM JG‐98 (Hsp70 inhibitor) for the indicated times. Tsc1 protein stability and Tsc2 protein stability were assessed by Western blotting.
-
C, DHEK293 cells were treated with either (C) 1 μM GB or (D) 2 μM SNX‐2112 (Hsp90 inhibitors) for the indicated times. Tsc1 protein stability and Tsc2 protein stability were assessed by immunoblotting. Akt and phospho‐S473‐Akt were used as positive controls.
-
EHEK293 cells were treated with 50 nM bortezomib (BZ) for the indicated times, and Tsc1 and Tsc2 proteins were evaluated by immunoblotting.
-
F50 nM BZ was added to HEK293 cells for 1 h followed by addition of 1 μM GB for 8 h. HEK293 cells were also treated individually with BZ or GB. UN represents untreated cells. Stability of Tsc2 was examined by immunoblotting.
-
GEmpty vector (EV) or Tsc2‐FLAG was used to transiently transfect HEK293 cells for 24 h followed by no treatment (UN), or treatment with either 50 nm BZ, 1 μM GB, or 10 μM JG‐98 for 4 h. Tsc2‐FLAG was immunoprecipitated, and ubiquitination was examined by immunoblotting with a anti‐pan‐ubiquitin antibody.
Source data are available online for this figure.
