Figure 3. Tsc1 facilitates the chaperoning of Hsp90 clients.
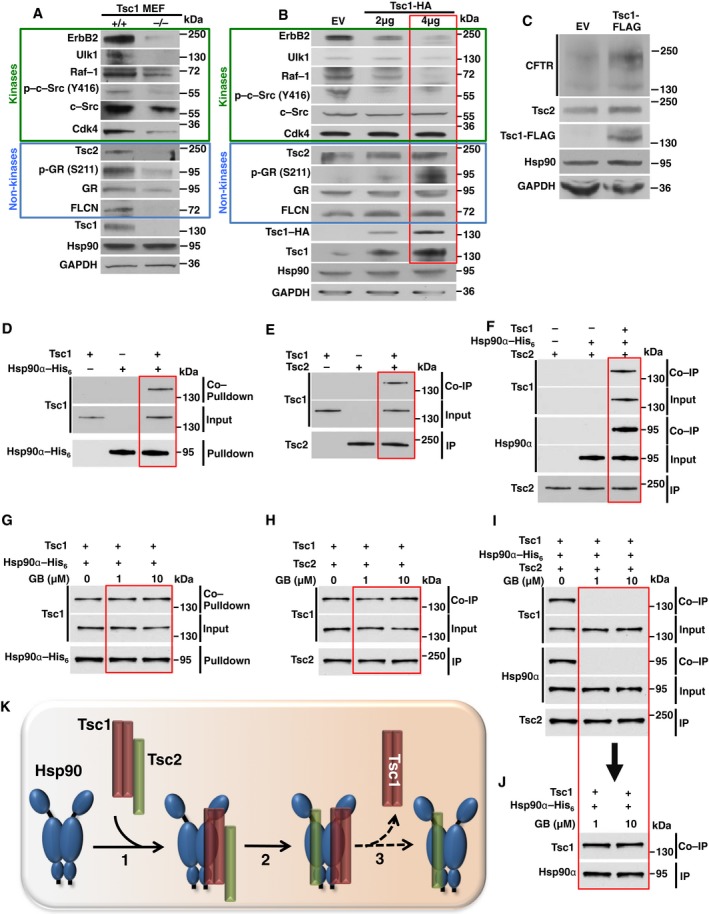
- Endogenous protein levels of Hsp90 kinase and non‐kinase clients from wild‐type and Tsc1−/− MEF cells were assessed by immunoblotting.
- Transient over‐expression of Tsc1‐HA in HEK293 cells and its impact on levels of Hsp90 clients was assessed by immunoblotting. Empty vector (EV) was used as a control.
- HEK293 cells were co‐transfected with CFTR and Tsc1‐FLAG. CFTR and Tsc1‐FLAG were detected by immunoblotting after 24 h. Empty vector (EV) was used as a control.
- Tsc1 interacts with Hsp90α‐His6 in vitro. Bacterially expressed and purified Hsp90α‐His6 was bound to Ni‐NTA agarose and then incubated with 10 ng pure Tsc1. Hsp90α‐His6 pulldown and Tsc1 co‐pulldown were examined by immunoblotting.
- Tsc1 binds to Tsc2 in vitro. Purified Tsc2 was bound to recombinant protein G agarose using anti‐Tsc2 antibody and then incubated with 10 ng pure Tsc1. Tsc2 immunoprecipitation and Tsc1 co‐immunoprecipitation were assessed by immunoblotting.
- Tsc1, Tsc2, and Hsp90α form a trimeric complex in vitro. Bacterially expressed and purified Hsp90α‐His6 was bound to Ni‐NTA agarose and followed by incubation with 10 ng Tsc1 and then 10 ng Tsc2 in vitro. Hsp90α‐His6 pulldown and Tsc1 and Tsc2 co‐pulldown were examined by immunoblotting.
- Samples from (D) were treated with indicated GB for 1 h at 4°C. Hsp90α‐His6 pulldown and Tsc1 co‐pulldown were examined by immunoblotting.
- Samples from (E) were treated with indicated GB for 1 h at 4°C. Tsc2 immunoprecipitation and Tsc1 co‐immunoprecipitation were assessed by immunoblotting.
- Samples from (F) were treated with indicated GB for 1 h at 4°C. Tsc2 immunoprecipitation and Tsc1 and Hsp90α co‐immunoprecipitation were assessed by immunoblotting.
- Supernatant from (I) was used to immunoprecipitate Hsp90α‐His6 and co‐immunoprecipitate Tsc1, which were examined by immunoblotting.
- Co‐chaperone Tsc1 binds to Tsc2 (1) and acts as a loading scaffold facilitating the direct binding of Tsc2 to Hsp90 (2), eventually leading to dissociation of Tsc1 from Hsp90 (3).
Source data are available online for this figure.
