Figure 7. Impact of Tsc1 co‐chaperone on the Hsp90 chaperone cycle.
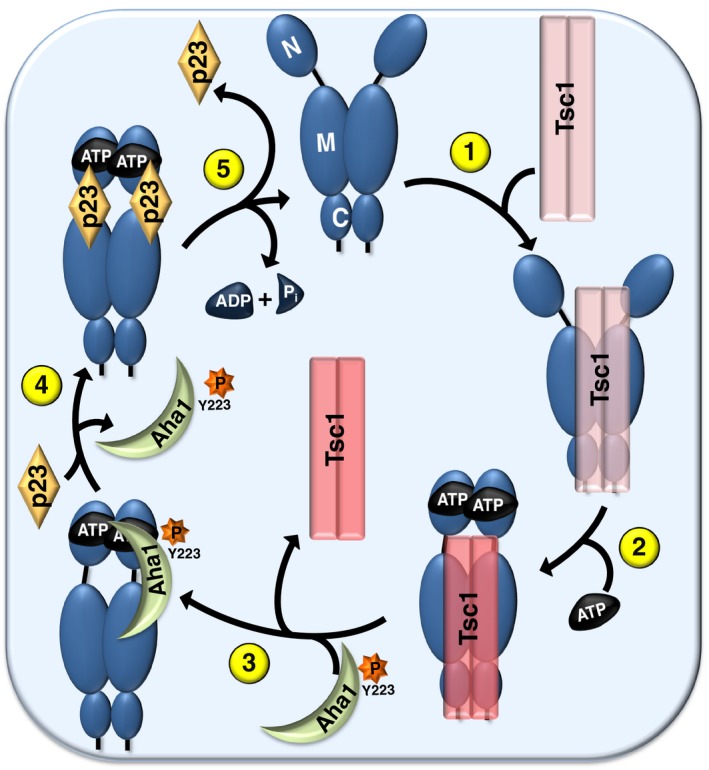
(1) Tsc1 binds weakly to the Hsp90 “open” conformation in the absence of nucleotides. (2) Upon ATP binding to the N‐domain of Hsp90, Tsc1 interaction strengthens to the “closed” conformation of Hsp90. (3) c‐Abl‐mediated phosphorylation of Aha1‐Y223 displaces Tsc1 from Hsp90. (4) Later in the chaperone cycle, p23 is predicted to expel phosphorylated Aha1‐Y223 from Hsp90, (5) allowing Hsp90 to hydrolyze ATP.
