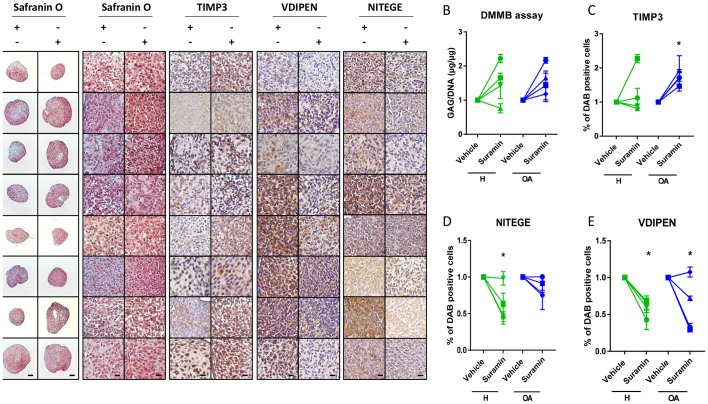
Figure 4.
Suramin’s chondroprotective effects and molecular mechanism via TIMP3 are present in human articular chondrocytes. (A) Safranin O staining of human articular chondrocyte pellets demonstrating increased proteoglycan content after suramin treatment compared with controls. Scale bars 100 µm, right panels: higher magnification of Safranin O staining and immunohistochemistry on human articular chondrocyte pellets. Scale bars 20 µm. (B) Di-methylmethylene blue (DMMB) assay demonstrating increased sulfated glycosaminoglycan content in human articular chondrocyte pellets after suramin treatment for 14 days compared with control (*P<0.05 for suramin treatment, two-way analysis of variance (ANOVA); P=0.14 and 0.07 for healthy and osteoarthritis (OA) cells, respectively, by Sidak test). (C) Quantitative assessment of immunohistochemical stainings demonstrating increased TIMP3 (*P<0.05 for suramin treatment, two-way ANOVA; P=0.5 and 0.03 for healthy and OA cells, respectively, by Sidak test) and reduced (D) VDIPEN (*P<0.01 for suramin treatment, two-way ANOVA; P=0.02 and 0.03 for healthy and OA cells, respectively, by Sidak test) and (E) NITEGE levels in suramin-treated human articular chondrocyte pellets relative to controls (*P<0.01 for suramin treatment, two-way ANOVA; P=0.005 and 0.31 for healthy and OA cells, respectively, by Sidak test). Individual data points are the means of three technical replicates per experiment.