Abstract
Cefepime is a fourth generation cephalosporin which is bactericidal for broad spectrum of organisms. This is a case-series of three patients who presented to our hospital with confusion secondary to cefepime use to treat urinary tract infection (UTI) and health care associated pneumonia (HCAP), after excluding other common etiologies of altered mental status (AMS). Of these three patients, one had progressive expressive aphasia and the other two demonstrated asynchronous myoclonic activity of the limbs. The symptoms were seen within four to five days of initiating the treatment and resolved within three days of discontinuation of cefepime. Acute structural abnormalities were excluded by computed tomography (CT) and magnetic resonance imaging (MRI) of the brain. Electroencephalogram (EEG) showed diffuse slowing activity with triphasic waves consistent with encephalopathy. In one patient, renal function was within normal limits, whereas it was abnormal in two patients. To our knowledge, this is the first report of cefepime induced asynchronous myoclonus and expressive aphasia in a patient with normal kidney function.
Keywords: Cefepime, Neurotoxicity, Seizures, Myoclonic activity, Expressive aphasia, Diffuse slowing, Impaired renal function
Highlights
-
•
Recognizing cefepime neurotoxicity could be challenging in acute care settings.
-
•
Caution is recommended in patients with renal and hepatic impairments.
-
•
Patients may present with expressive aphasia and/or myoclonus even with normal kidney function.
-
•
Fortunately in most cases, neurotoxicity is reversible upon discontinuation of cefepime.
1. Introduction
Cefepime is a 4th generation cephalosporin antibiotic commonly used in the inpatient setting. Clearance primarily occurs via the kidney and neurotoxicity with a range of neurological manifestations has been reported particularly in patients with renal impairment. Cefepime induced neurotoxicity can be challenging to recognize but it is generally reversible. In this case series, we present three patients who developed cefepime induced neurotoxicity and experienced complete recovery with discontinuation.
Jallon et al. described 19 cases of severe encephalopathy associated with renal impairment [1]. Prolonged confusion with temporospatial disorientation and hallucinations persisted in these patients for 2–8 days after cefepime discontinuation. Bilateral distal myoclonus was also reported in a quarter of the cases. Chow et al. reviewed 42 cases and reported confusion and temporospatial disorientation in 96% of the cases, myoclonus in 33% and seizures in 13% [2]. Many of these patients were elderly with uremia. Median of cefepime treatment before symptom onset was 5 days. The median time lag between symptom onset and diagnosis was also 5 days, which emphasizes the need for improved awareness regarding cefepime neurotoxicity.
There are also less frequent reports of neurotoxicity despite renal adjustments to dosage. Gangireddy et al. described a febrile neutropenic patient with stage IV chronic kidney disease who was treated with renally dosed cefepime for pneumonia [3].
2. Results
We present three cases of cefepime induced neurotoxicity in whom the diagnosis was made after excluding other common etiologies of AMS.
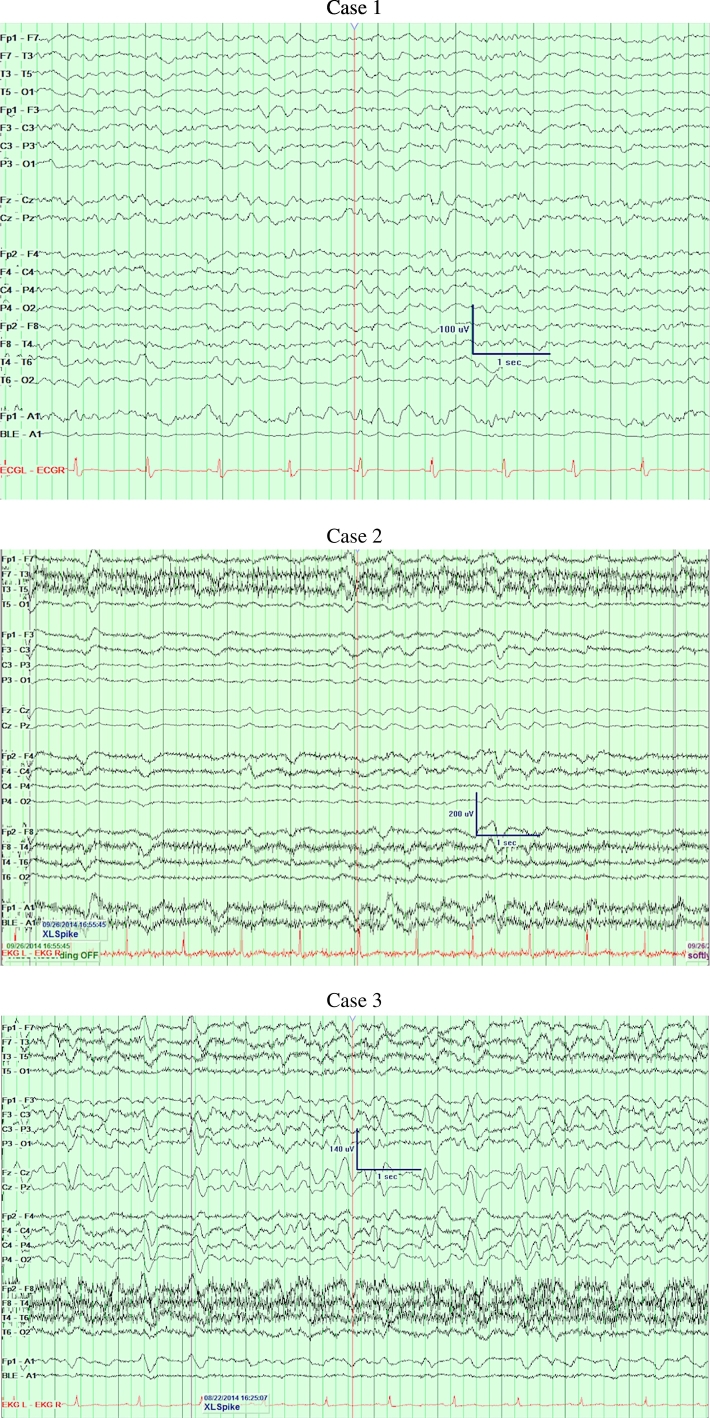
In one patient, neurotoxicity was manifested by new onset progressive expressive aphasia which was seen within four days of initiating treatment and was not reported at the time of initial presentation. Two patients demonstrated asynchronous myoclonic activity of the limbs (see Table 1). The symptoms were seen within four to five days of initiating the treatment. The symptoms resolved completely within three days of discontinuation of cefepime in all patients. Acute structural abnormalities were excluded by CT and/or MRI of the head (see Fig. 1). EEG showed diffuse slowing activity with classical triphasic morphology and prominent positive phase most consistent with toxic/metabolic encephalopathy (see Fig. 2). In one patient renal function was within normal limits, whereas it was abnormal in two patients (see Table 2).
Table 1.
Summary of semiological findings of clinical events.
| Myoclonic activity | |
|---|---|
| Case 1 | Asynchronous myoclonic jerks of all 4 extremities |
| Case 2 | Asynchronous myoclonic jerks, arm twitching, lip smacking |
| Case 3 | Increased left upper extremity tone with contracture and no withdrawal (w/d) to nail bed pressure (NBP), there is w/d to NBP in RUE |
Fig. 1.
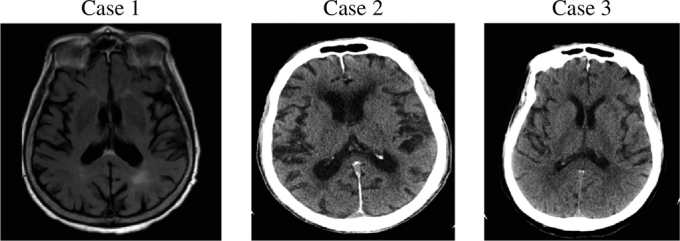
Images.
Case 1 – MRI Brain axial fluid-attenuated inversion recovery (FLAIR) view: Chronic small vessel disease, no acute abnormality.
Case 2 – Head CT: Global volume loss, chronic small vessel disease, no acute abnormality.
Case 3 – Head CT: Chronic small vessel disease, no acute abnormality.
Fig. 2.
EEGs.
Case 1 – Diffuse slowing.
Case 2 – Diffuse slowing.
Case 3 – Diffuse moderate to severe background slowing with triphasic waves.
Table 2.
Summary of demographics.
| Age/sex | Creatinine levels (0.5–1.1 mg/dl) | Indication for Cefepime | Neurological symptom onset | |
|---|---|---|---|---|
| Case 1 | 86/F | Cr 0.8 | Urinary tract infection | Day 4 |
| Case 2 | 88/M | Cr 2.16 (b/l 1.5–2.4) | Healthcare associated pneumonia | Day 5 |
| Case 3 | 64/F | Cr 1.7 (b/l 1.5–2.0) | Urinary tract infection | Day 5 |
3. Discussion
We present a case-series of 3 patients with cefepime induced neurotoxicity. To our knowledge, this is the first report of cefepime induced asynchronous myoclonus and expressive aphasia in patients with normal kidney function. Neurotoxicity in patients with or without renal failure who are treated with cefepime has been reported. Neurotoxicity is an underreported side effect of cefepime especially in patients with normal renal function [4].
Besides impaired kidney function, other established risk factors that should be considered include advanced patient age, liver disease and pre-existing neurological disorders [3]. Neurotoxicity has been reported for other antibiotics and cephalosporins but there is evidence of increased risk with cefepime [5].
Perhaps, this increased risk reflects enhanced affinity for the GABA receptor due to specific molecular conformations of the drug. Alternatively, cefepime may exhibit chemical structural affinity for increased accumulation in spinal fluid.
Retrospective study by Fugate et al. identified 15 cases out of 100 ICU patients [4]. Patients were treated with cefepime for at least 3 days before developing symptoms including impaired consciousness, encephalopathy, and myoclonus. The authors stratified the strength of association between symptoms and cefepime use as 7 cases being considered definite, 3 probable and 5 possible. This effort to stratify likelihood reflects the frequent cloudiness of the clinical picture in patients requiring cefepime.
Another retrospective analysis of 30 febrile neutropenic patients by Lamoth et al. aimed to determine the association between plasma cefepime levels and associated neurotoxicity [6]. Out of the 30 cases, 6 developed neurological symptoms that improved or resolved completely with cefepime discontinuation. High serum concentration of cefepime was an independent predictor of neurotoxicity with a 50% probability threshold at concentrations greater than 22 mg/l. Importantly the authors noted all 6 cases displayed only mild renal impairment with GFRs ranging from 41 to 65 ml/min. This study demonstrated the utility of serum measurements thereby providing justification for greater accessibility to such testing.
In 2012, the FDA issued a Safety Warning reminding of the need to make adjustments to cefepime dosage based on renal function [7]. Statement referenced 59 cases of non-convulsive status epilepticus reported in the FDA's Adverse Event Reporting System database between 1996 and 2012.
In summary, recognizing cefepime as a source of neurotoxicity can be a challenge given the clinical picture is often clouded by accompanying causes of encephalopathy such as metabolic disturbances, infection, uremia, and hepatic encephalopathy. For this reason, a heightened index of suspicion is required when treating patients with renal and hepatic impairments even when cefepime is dosed appropriately. This report highlights atypical presentation of cefepime induced toxicity with focal neurological symptoms despite absence of structural abnormalities on MRI of the brain and the possibility of occurrence of cefepime induced neurotoxicity even with normal renal and liver function.
Authors' roles
Cigdem Isitan, M.D.: Conception, data collection, organization, manuscript preparation.
Andrew Ferree, M.D.: Data collection, organization, manuscript preparation.
Anna Depold Hohler, M.D.: Review, critique and supervision.
Disclosures
No specific funding was received for this work and authors declare that there are no conflicts of interest relevant to this work.
The authors declare that there are no additional disclosures to report.
IRB approval
Study protocol has been approved by our institute's committee on human research with IRB Number: H-34519. It was determined that the study qualifies for an exemption determination under the policies and procedures of the Human Research Protection Program. Approval letter will be provided upon request for further details.
Contributor Information
Cigdem Isitan, Email: cigdem.isitan@yale.edu.
Andrew Ferree, Email: andrew.ferree@bmc.org.
Anna DePold Hohler, Email: anna.hohler@bmc.org.
References
- 1.Jallon P., Fankhauser L., Du Pasquier R. Severe but reversible encephalopathy associated with cefepime. Neurophysiol. Clin. 2000;30(6):383–386. doi: 10.1016/s0987-7053(00)00234-3. [DOI] [PubMed] [Google Scholar]
- 2.Chow K.M., Szeto C.C., Hui A.C. Retrospective review of neurotoxicity induced by cefepime and ceftazidime. Pharmacotherapy. 2003;23(3):369–373. doi: 10.1592/phco.23.3.369.32100. [DOI] [PubMed] [Google Scholar]
- 3.Gangireddy V.G., Mitchell L.C., Coleman T. Cefepime neurotoxicity despite renal adjusted dosing. Scand. J. Infect. Dis. 2011;43(10):827–829. doi: 10.3109/00365548.2011.581308. [DOI] [PubMed] [Google Scholar]
- 4.Fugate J.E., Kalimullah E.A., Hocker S.E. Cefepime neurotoxicity in the intensive care unit: a cause of severe, underappreciated encephalopathy. Crit. Care. 2013;17(6):R264. doi: 10.1186/cc13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka A., Takechi K., Watanabe S. Comparison of the prevalence of convulsions associated with the use of cefepime and meropenem. Int. J. Clin. Pharm. 2013;35(5):683–687. doi: 10.1007/s11096-013-9799-3. [DOI] [PubMed] [Google Scholar]
- 6.Lamoth F., Buclin T., Pascual A. High cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients with mild impairment of renal function. Antimicrob. Agents Chemother. 2010;54(10):4360–4367. doi: 10.1128/AAC.01595-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA's Adverse Event Reporting System (AERS) database, from the date of approval of cefepime in 1996 through February 2012.