Abstract
Periapical lesions of endodontic origin are common pathological conditions affecting periradicular tissues. Microbial infection of pulpal tissues is primarily responsible for initiation and progression of apical periodontitis. The primary objective of endodontic therapy should be to restore involved teeth to a state of normalcy nonsurgically. Different nonsurgical management techniques, namely, conservative root canal therapy, decompression technique, method using calcium hydroxide, aspiration-irrigation technique, lesion sterilization and tissue repair therapy, active nonsurgical decompression technique, and the apexum procedure have been advocated. New techniques which use drug-loaded injectable scaffolds, simvastatin, and epigallocatechin-3-gallate have been tried. Surgical option should be considered when intra- or extra-radicular infections are persistent. Incidence of nonendodontic periapical lesions has also been reported. An accurate diagnosis of the periapical lesion whether it is of endodontic or nonendodontic origin has to be made. Surgical methods have many disadvantages, and hence should be considered as an option only in the case of failure of nonsurgical techniques. Assessment of healing of periapical lesions has to be done periodically which necessitates a long-term follow-up. Even large periapical lesions and retreatment cases where the lesion is of endodontic origin have been successfully managed nonsurgically with orthograde endodontic therapy.
Keywords: Nonsurgical management, orthograde approach, periapical lesions
INTRODUCTION
Periapical lesions are one of the common pathological conditions affecting periradicular tissues. The microbial invasion and subsequent infection of the canal systems of root play a decisive role in the initiation and progression of periapical lesions.[1] A well-planned and executed access, biomechanical preparation of root canal system followed by three-dimensional obturation contributes to successful treatment outcomes. The complete elimination of microorganisms and their by-products from within the canal system and preventing reinfection are of utmost importance in the management of periapical pathology of endodontic origin.
The primary objective of endodontic therapy should be to restore involved teeth to a state of normalcy using nonsurgical management techniques. All inflammatory periapical lesions should be initially treated with conservative procedures.[2] Large periapical lesions and apical true cysts are of inflammatory origin and should be treated initially with a nonsurgical approach.[3] When intra- or extra-radicular infections are persistent, and periapical pathology fails to resolve after nonsurgical endodontic management protocols, only then a surgical option should be considered.[4]
Microorganisms within canal systems should be eradicated for successful outcomes of root canal therapy.[5] Different techniques can be used in the nonsurgical management of periapical pathologies, namely, orthograde root canal therapy, decompression technique, method using calcium hydroxide, aspiration-irrigation technique, lesion sterilization and repair therapy, active nonsurgical decompression technique and the apexum procedure. Surgical methods have many disadvantages, and hence should be considered as an option only in the case of failure of nonsurgical techniques.[6]
Host defenses are not capable of eradicating the infection established in the necrotic pulp because of a lack of circulation to the areas of pulpal necrosis. The immune response of the periapical tissues includes both innate and adaptive responses to irritants. Pathological changes in periapical tissues are usually not because of microorganisms themselves but rather due to by-products of microbes and toxins that diffuse though inflamed pulpal tissue to the periapical region as the microbial infection gradually progresses within the canal system of the roots toward the apex.[7,8,9] Factors such as proper diagnosis, extent of lesion, proximity to adjacent vital teeth, general health status, presence of canal obstructions, and time required for therapy have to be considered before deciding on the management methodologies. An accurate diagnosis of the periapical lesion and whether it is of endodontic origin has to be made. Nonendodontic periapical lesions have also been reported.[10,11] Even large periapical lesions have been successfully managed nonsurgically.[12]
CASE REPORTS
Case 1
A 19-year-old male reported with pain and swelling in relation to upper anterior teeth. A history of an accident 2 years back involving trauma to teeth was recorded. Oral examination revealed discolored left upper central incisor (21) and lateral incisor (22), with a mild tender periapical swelling in relation to 21 and 22. No anatomical malformation of incisors or sinus was present. On radiographic examination, 11 was root treated, a radiolucency was seen in relation to 21 and 22 periapically [Figure 1a]. Electronic pulp testing (Electric Pulp Tester, Parkell, Farmingdale, NY) and cold application (ice stick) were negative for 21 and 22. Based on these findings, a provisional diagnosis of a necrotic pulp with chronic periradicular periodontitis involving 21 and 22 was made. The patient was informed of the long-term prognosis, and orthograde nonsurgical endodontic therapy was planned. At the same appointment, root canal therapy was initiated on 21 and 22, canals instrumented with size 15–80 K-files using a step-back technique. The canal was irrigated copiously with 2.5% sodium hypochlorite (NaOCl) solution using a 28-gauge endodontic side vent needle after each instrument. After rinsing with normal saline, the final irrigation was done with 17% ethylenediaminetetraacetic acid (EDTA) solution, canal dried with sterile paper points. Calcium hydroxide paste was loaded in canals using a lentulo spiral and was replaced every 2 weeks for 3 months. The access cavity was sealed with zinc oxide eugenol cement after each visit. After 3 months, the tooth remained asymptomatic, obturated with gutta-percha, and AH Plus using a lateral condensation technique and access cavity was sealed with composite resin. 11 was also retreated. Radiographic examination after 6 months showed previous radiolucent lesion had decreased in size. After 1 year, there was significant amount of healing with the formation of new trabecular bone [Figure 1].
Figure 1.
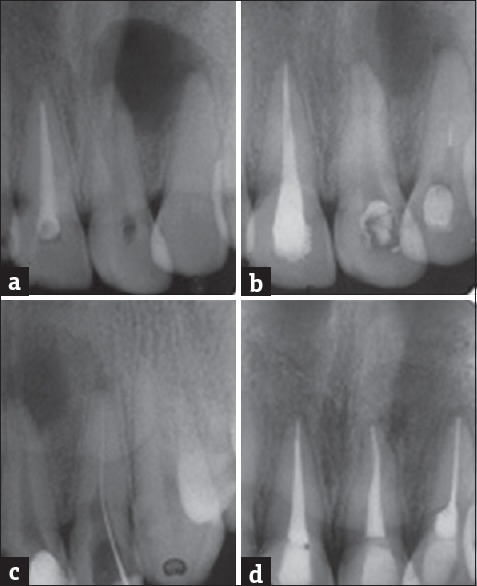
(a) Preoperative radiograph showing periapical lesion involving both roots of 21, 22. (b) 11 retreated, access preparation on 21, 22 establishing drainage. (c) Diagnostic radiograph. (d) One-year review showing formation of new bone
Case 2
A 17-year-old male reported with pain and mild swelling in relation to left upper anterior teeth and maxillary region. Intraoral examination revealed that upper left lateral incissor was discolored and there was a mild, tender swelling in relation to the periapical region of the tooth. The tooth was not mobile; there was negative response to heat test and there was no response to electric pulp testing (Electric Pulp Tester, Parkell, Farmingdale, NY, USA). On radiographic examination, a radiolucency was seen in relation to 21 and 22 involving both the apices. 21 gave a mild response to electric pulp test and the tooth was not discolored. Clinically, no anatomical malformation of the incisors was detected. A provisional diagnosis of a necrotic pulp with chronic periradicular periodontitis of 21 and 22 was made and endodontic treatment scheduled. The patient was informed of long-term prognosis and a decision was made for conservative nonsurgical management of 21 and 22. At the same appointment, root canal treatment was initiated on both teeth. Necrotic pulp tissue was extirpated, and the canal was instrumented with size 15–80 K-files using a step-back technique. During the instrumentation, the canal was irrigated copiously with 2.5% NaOCl solution using a 27-gauge endodontic needle after each instrument followed by normal saline. The final irrigation 17% EDTA solution. The canal was dried with sterile paper points and initially filled with calcium hydroxide paste, which was changed every 2 weeks for 3 months and the access cavity was sealed. The teeth were asymptomatic and subsequently obturated with gutta-percha cones and AH 26 sealer using cold lateral condensation technique, and entrance filling done. The teeth remained asymptomatic at 12 months and radiological examination revealed significant healing, with the previous radiolucent lesion size reduced and formation of trabecular bone [Figure 2].
Figure 2.
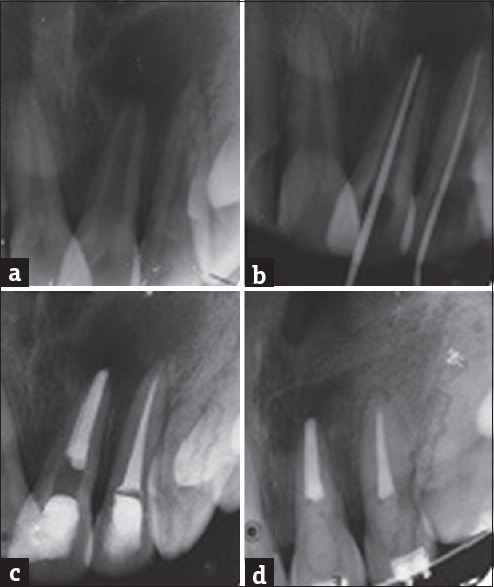
(a) Preoperative radiograph showing periapical lesion involving both roots of 21, 22. (b) Master cone. (c) Immediate postobturation. (d) One-year review showing formation of new bone
Case 3
A 19-year-old female patient reported to the department of conservative dentistry with a complaint of gradually worsening pain in relation to lower left molar region for 2 weeks. There was sharp intermittent radiating pain which aggravated on chewing and postural changes. Intraoral examination revealed a deep class I carious lesion on the left mandibular first (36) and second molar (37). The tooth was not mobile, tender to percussion and there was a swelling in the buccal aspect in relation to periapical region of 36. The tooth gave a negative response to thermal and electric pulp testing and was nonvital. Radiographic examination revealed a radiolucency in the periapical region of 36 on the both the mesial and the distal root. Based on these findings, a provisional diagnosis of necrotic pulp and chronic apical periodontitis in the left mandibular first molar was made. Although the caries was deep in 37, the tooth was asymptomatic. Emergency endodontic management was done on 36. Conservative nonsurgical management was planned and root canal therapy initiated on 36 under local anesthesia with rubber dam isolation. The carious lesion removed, adequate access preparation done, and three canals were identified, instrumented, and enlarged with size 10–20 K-files (Dentsply, Maillefer, Ballaigues, Switzerland) using a step-back technique. After establishment of a glide path, the biomechanical preparation was done using ProTaper rotary instruments (21 mm - S1, S2, F1, F2, F3 - Dentsply Maillefer, Ballaigues, Switzerland). The working length of the canals was confirmed using an apex locator (Raypex 6, VDW, Munich, Germany). During instrumentation, the canals were irrigated copiously with 2.5% NaOCl solution (Nice chemicals Pvt Ltd., India) using a 28-gauge endodontic side vent needle after each instrument followed by normal saline (Nirlife Health Care, Nirma Products, India). Final irrigation was done with 17% EDTA solution (pulpdent Corporation, USA). The canal was dried with sterile paper points. Calcium hydroxide powder was mixed with sterile distilled water and loaded into the canals using a lentulo spiral. This was changed every 2 weeks for a period of 3 months and the tooth remained asymptomatic. Subsequently the canals were cleansed and obturated with gutta-percha cones (Dentsply, Maillefer, Ballaigues, Switzerland) and AH Plus sealer (Dentsply DeTrey, GmbH, Konstanz, Germany) using a cold lateral condensation technique. As the cavity was deep in 37 and the periapical region showed changes that root canal therapy was initiated under local anesthesia, calcium hydroxide dressings changed every 2 weeks and subsequently obturated. The patient experienced no postoperative sequelae and postendodontic restoration was given to ensure adequate coronal seal. Radiographic examination after 6 months revealed adequate healing with formation of trabecular bone. The 12-month review showed a significant amount of healing with resolution of the periapical lesion [Figure 3].
Figure 3.
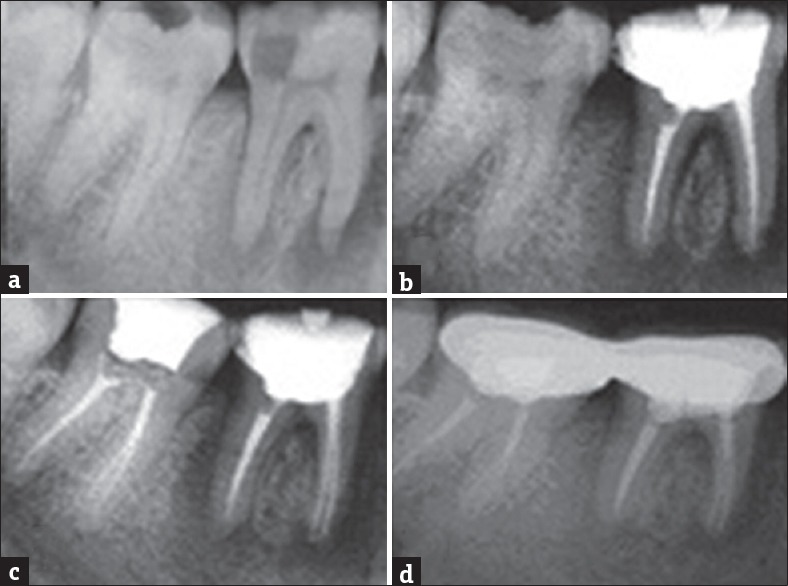
(a) Preoperative radiograph showing periapical lesion involving both roots of 36, 37. (b) Immediate postobturation radiograph 36. (c) Immediate postobturation radiograph 37. (d) One-year review showing formation of new bone
DISCUSSION
Periapical lesions encountered in endodontic practice result from apical periodontitis due to root canal space infection and are a response of the host defense against the microbial action. The progression of this lesion results in local inflammation, resorption of hard tissues, and destruction of other periapical tissues. The preliminary diagnosis of a chronic periapical lesion is based on clinical symptoms and subsequent radiological investigations. A histological examination of the lesion is done to confirm the exact nature of the lesion. There have also been reports of lesions in the periapical region which are of nonendodontic origin.[13,14]
Periapical lesions of endodontic origin are discovered on routine radiographic examination. They are commonly periapical cysts, granulomas, or abscesses. A fragile equilibrium exists between host defense mechanisms and microorganisms. A change can give way to intense variations from the type of pathogens which proliferate to the kind of pathologies encountered. Inflammatory response of host defense mechanisms to the presence of microorganisms and microbial byproducts in the root canal space eventually leads to apical periodontitis.
The development apical periodontitis is protective and thus aimed at preventing microorganisms from leaving the root of the tooth and spreading systemically. However, it is also destructive. The same biomolecules and host defense cells that are capable of destroying and damaging microbial cells are also able to exert the same effect on host cells. The objective of curative intervention in endodontic therapy is to eliminate, or significantly reduce, the number of microbial organisms colonizing the root canal system. The natural balance between host response and microorganisms in the root canal space if set right by a reduction in the number of microorganisms results in healing of periapical tissues.
A thorough clinical examination, radiological investigation, analysis of past and present medical history, pulp vitality tests, and aspiration are essential tools for developing a correct diagnosis of periapical lesions.[11,12,15] The nonendodontic periapical lesions can be categorized into benign or malignant, mimicking lesions of endodontic origin. Majority of the lesions reported were benign in nature and were either ameloblastomas, giant cell lesions, central fibroma, central hemangioma, inflammatory bone disease, traumatic bone cyst, odontogenic keratocyst, dentigerous cyst, fibrous dysplasia, nasopalatine duct cysts, basal cell nevus syndrome, and the brown tumor of hyperparathyroidism or stafne bone cavities.[13,14,16,17] Malignant lesions were metastatic lesions or carcinomas. If the investigations discussed earlier point to a lesion of nonendodontic origin, a biopsy with a histopathological analysis is mandatory.
A number of studies have reported a success rate of up to 85 percent after endodontic treatment of teeth with periapical lesions.[18,19,20] All inflammatory periapical lesions should be initially treated with conservative nonsurgical management techniques. Various techniques can be successfully used in the nonsurgical management of periapical lesions such as conservative root canal therapy, method using calcium hydroxide, aspiration-irrigation technique, the decompression technique, lesion sterilization and repair therapy, active nonsurgical decompression technique, the apexum procedure, and the use of injectable scaffolds. Periodic monitoring of healing process of the periapical lesion post nonsurgical management is mandatory.[9] The selection of the nonsurgical methodology should be based on the type of the periapical lesion present.
Bhaskar's hypothesis proposed that instrumentation beyond the confines of the apical foramen leads to transitory inflammation and ulceration of the epithelial lining leading to resolution of periapical cysts.[21] This establishes effective drainage and relives pressure. On cessation of drainage, fibroblast proliferation leads to new collagen formation compressing the capillary network which leads to degeneration of the starved epithelial cells which are engulfed by the macrophages.[22] The drainage of cystic fluid which contains cholesterol crystals, drying of the canals, and weekly debridement over a period of 2–3 weeks, followed by obturation has led to a regression of the lesion and complete resolution of lesions within a year.[23]
When the canals are draining cystic fluid the aspiration–irrigation and decompression techniques can be used. These techniques act by decreasing the hydrostatic pressure within periapical lesions and when there is no drainage of fluid from canals, calcium hydroxide, or the triple antibiotic paste is typically changed every few weeks and follow-up done until healing of the lesion. Establishing apical patency is vital in nonsurgical management of these lesions.
Injectable in situ forming scaffold-loaded with risedronate, a bone resorption inhibitor, and lornoxicam, an anti-inflammatory drug has been tried in the nonsurgical treatment of periapical lesions. The scaffold technique succeeded in enhancing the formation of new bone and reduced inflammation confirming success of use of prepared scaffolds as an innovative approach in the treatment of bone defects of the periapical region.[24]
Cysteine-rich 61 (Cyr61) and CCL2, a chemokine responsible for macrophage chemotaxis, are potential osteolytic mediators in inflammatory bone diseases. Osteoblastic expression of Cyr61 correlates with the severity of bone loss in periapical lesions, but the regulatory mechanism of Cyr61 expression was not known. Major histocompatibility complex class II transactivator is a repressor of Cyr61 synthesis in osteoblasts and might play a protective role in the pathogenesis of bone resorption in apical periodontitis, possibly through downregulating the expression of Cyr61 in osteoblasts.[25] MicroRNA-335-5p is an inflammatory mediator and may have dual roles in the progression of apical periodontitis, and the relevant regulation mechanism is complex. MicroRNA-335-5p could be a potential therapeutic target in apical periodontitis.[26]
Recent investigations indicate that hydroxymethylglutaryl-coenzyme A reductase inhibitors widely used for cholesterol lowering properties, and epigallocatechin-3-gallate (EGCG), the major polyphenol of green tea, have anti-inflammatory properties. Simvastatin and EGCG suppress the progression of apical periodontitis, possibly by diminishing Cyr61 expression in osteoblasts and subsequent macrophage chemotaxis into the periapical lesions.[27,28] Simvastatin may also alleviate periapical lesions by enhancing transcription factor Forkhead/winged helix box protein O3a to suppress the synthesis of Cyr61 in osteoblasts.[29]
The role of interleukin (IL)-6 in apical periodontitis pathogenesis as a pro-inflammatory cytokine and whether it could be used as a biomarker that can predict the progression of bone resorption has been evaluated. In apical pathologies, IL-6 levels were higher in symptomatic, epithelialized, and large lesions than in asymptomatic and small lesions. In the presence of infections, increasing IL-6 levels leads to bone resorption. Other cytokines such as IL-1 and tumor necrosis factor-alpha induce bone resorption when IL-6 is not present.[30]
Periodic recall radiographs, radiographic assessment of the lesion using Strindberg criteria,[31] the periapical index,[32] and the color ultrasound Doppler[33] are methods that have been used to assess the healing of periapical lesions. Healing of larger lesions could be assessed using tomographic imaging techniques. After the elimination or reduction of microorganisms, the inflammatory cytokines and mediators within the lesion undergo a shift from a proinflammatory, destructive nature to an anti-inflammatory, and proliferative nature. Osteoprogenitor cells on the periphery of the lesion then undergoes differentiation into osteoblasts which are capable of secreting new bony matrix to replace the bone lost by the lesion of apical periodontitis. The normal balance between bone forming osteoblasts and bone resorbing osteoclasts shifts toward an increase in bone formation during healing. Posttreatment, when the lesion is asymptomatic, it can also undergo fibrous healing or healing by formation of a scar. Radiographic examination reveals trabecular bone pattern radiating from the center but appears as a incompletely resolved radiolucency.[34]
CONCLUSION
A nonsurgical approach should always be adopted as a routine measure in periapical lesions of endodontic origin. Conservative orthograde endodontic therapy demonstrates favorable outcomes. Regular periodic review and assessment of the healing process of periapical lesions are essential. The surgical approach can be adopted for cases where canal is non-negotiable, for cases refractory to nonsurgical management techniques, cases where long-term periodic monitoring of periapical lesions is not feasible and lesions of nonendodontic origin.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Croitoru IC, CraiToiu S, Petcu CM, Mihailescu OA, Pascu RM, Bobic AG, et al. Clinical, imagistic and histopathological study of chronic apical periodontitis. Rom J Morphol Embryol. 2016;57(2 Suppl):719–728. [PubMed] [Google Scholar]
- 2.Lin LM, Huang GT, Rosenberg PA. Proliferation of epithelial cell rests, formation of apical cysts, and regression of apical cysts after periapical wound healing. J Endod. 2007;33:908–16. doi: 10.1016/j.joen.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Santos Soares SM, Brito-Júnior M, de Souza FK, Zastrow EV, Cunha CO, Silveira FF, et al. Management of cyst-like periapical lesions by orthograde decompression and long-term calcium hydroxide/chlorhexidine intracanal dressing: A case series. J Endod. 2016;42:1135–41. doi: 10.1016/j.joen.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Lin LM, Ricucci D, Lin J, Rosenberg PA. Nonsurgical root canal therapy of large cyst-like inflammatory periapical lesions and inflammatory apical cysts. J Endod. 2009;35:607–15. doi: 10.1016/j.joen.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Salamat K, Rezai RF. Nonsurgical treatment of extraoral lesions caused by necrotic nonvital tooth. Oral Surg Oral Med Oral Pathol. 1986;61:618–23. doi: 10.1016/0030-4220(86)90107-6. [DOI] [PubMed] [Google Scholar]
- 6.Sood N, Maheshwari N, Gothi R, Sood N. Treatment of large periapical cyst like lesion: A noninvasive approach: A report of two cases. Int J Clin Pediatr Dent. 2015;8:133–7. doi: 10.5005/jp-journals-10005-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricucci D, Pascon EA, Ford TR, Langeland K. Epithelium and bacteria in periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:239–49. doi: 10.1016/j.tripleo.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes M, de Ataide I. Nonsurgical management of periapical lesions. J Conserv Dent. 2010;13:240–5. doi: 10.4103/0972-0707.73384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirotheau Corrêa Pontes F, Paiva Fonseca F, Souza de Jesus A, Garcia Alves AC, Marques Araújo L, Silva do Nascimento L, et al. Nonendodontic lesions misdiagnosed as apical periodontitis lesions: Series of case reports and review of literature. J Endod. 2014;40:16–27. doi: 10.1016/j.joen.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Huang HY, Chen YK, Ko EC, Chuang FH, Chen PH, Chen CY, et al. Retrospective analysis of nonendodontic periapical lesions misdiagnosed as endodontic apical periodontitis lesions in a population of Taiwanese patients. Clin Oral Investig. 2017;21:2077–82. doi: 10.1007/s00784-016-1997-7. [DOI] [PubMed] [Google Scholar]
- 12.Oztan MD. Endodontic treatment of teeth associated with a large periapical lesion. Int Endod J. 2002;35:73–8. doi: 10.1046/j.1365-2591.2002.00455.x. [DOI] [PubMed] [Google Scholar]
- 13.Branstetter BF, Weissman JL, Kaplan SB. Imaging of a Stafne bone cavity: What MR adds and why a new name is needed. AJNR Am J Neuroradiol. 1999;20:587–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlkemper P, Wolcott JF, Pringle GA, Hicks ML. Periapical central giant cell granuloma: A potential endodontic misdiagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:739–45. doi: 10.1067/moe.2000.108803. [DOI] [PubMed] [Google Scholar]
- 15.Sena Guimarães AL, Marques-Silva L, Cavaliéri Gomes C, Castro WH, Mesquita RA, Gomez RS. Peripheral brown tumour of hyperparathyroidism in the oral cavity. Oral Oncol Extra. 2006;42:91–3. [Google Scholar]
- 16.Bitar GJ, Herman CK, Dahman MI, Hoard MA. Basal cell nevus syndrome: Guidelines for early detection. Am Fam Physician. 2002;65:2501–4. [PubMed] [Google Scholar]
- 17.Garlock JA, Pringle GA, Hicks ML. The odontogenic keratocyst: A potential endodontic misdiagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:452–6. doi: 10.1016/s1079-2104(98)90073-7. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes M, De Ataide I. Non-surgical management of a large periapical lesion using a simple aspiration technique: A case report. Int Endod J. 2010;43:536–42. doi: 10.1111/j.1365-2591.2010.01719.x. [DOI] [PubMed] [Google Scholar]
- 19.Soares J, Santos S, Silveira F, Nunes E. Nonsurgical treatment of extensive cyst-like periapical lesion of endodontic origin. Int Endod J. 2006;39:566–75. doi: 10.1111/j.1365-2591.2006.01109.x. [DOI] [PubMed] [Google Scholar]
- 20.Al-Kandari AM, al-Quoud OA, Gnanasekhar JD. Healing of large periapical lesions following nonsurgical endodontic therapy: Case reports. Quintessence Int. 1994;25:115–9. [PubMed] [Google Scholar]
- 21.Bhaskar SN. Nonsurgical resolution of radicular cysts. Oral Surg Oral Med Oral Pathol. 1972;34:458–68. doi: 10.1016/0030-4220(72)90325-8. [DOI] [PubMed] [Google Scholar]
- 22.Bender IB. A commentary on General Bhaskar's hypothesis. Oral Surg Oral Med Oral Pathol. 1972;34:469–76. doi: 10.1016/0030-4220(72)90326-x. [DOI] [PubMed] [Google Scholar]
- 23.Salaria SK, Kamra S, Ghuman SK, Sharma G. Nonsurgical endodontic therapy along with minimal invasive treatment utilizing Bhasker's hypothesis for the management of infected radicular cystic lesion: A rare case report. Contemp Clin Dent. 2016;7:562–5. doi: 10.4103/0976-237X.194098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shamma RN, Elkasabgy NA, Mahmoud AA, Gawdat SI, Kataia MM, Abdel Hamid MA. Design of novel injectable in-situ forming scaffolds for non-surgical treatment of periapical lesions: In-vitro and in-vivo evaluation. Int J Pharm. 2017;521:306–17. doi: 10.1016/j.ijpharm.2017.02.058. [DOI] [PubMed] [Google Scholar]
- 25.Lee YL, Lin SK, Hong CY, Wang JS, Yang H, Lai EH, et al. Major histocompatibility complex Class II transactivator inhibits cysteine-rich 61 expression in osteoblastic cells and its implication in the pathogenesis of periapical lesions. J Endod. 2010;36:1021–5. doi: 10.1016/j.joen.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Yue J, Wang P, Hong Q, Liao Q, Yan L, Xu W, et al. MicroRNA-335-5p Plays Dual Roles in Periapical Lesions by Complex Regulation Pathways. J Endod. 2017;43:1323–8. doi: 10.1016/j.joen.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Lin SK, Kok SH, Lee YL, Hou KL, Lin YT, Chen MH, et al. Simvastatin as a novel strategy to alleviate periapical lesions. J Endod. 2009;35:657–62. doi: 10.1016/j.joen.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Lin LD, Lin SK, Chao YL, Kok SH, Hong CY, Hou KL, et al. Simvastatin suppresses osteoblastic expression of Cyr61 and progression of apical periodontitis through enhancement of the transcription factor Forkhead/winged helix box protein O3a. J Endod. 2013;39:619–25. doi: 10.1016/j.joen.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Lee YL, Hong CY, Kok SH, Hou KL, Lin YT, Chen MH, et al. An extract of green tea, epigallocatechin-3-gallate, reduces periapical lesions by inhibiting cysteine-rich 61 expression in osteoblasts. J Endod. 2009;35:206–11. doi: 10.1016/j.joen.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Azuma MM, Samuel RO, Gomes-Filho JE, Dezan-Junior E, Cintra LT. The role of IL-6 on apical periodontitis: A systematic review. Int Endod J. 2014;47:615–21. doi: 10.1111/iej.12196. [DOI] [PubMed] [Google Scholar]
- 31.Strindberg LZ. The dependence of the results of pulp therapy on certain factors. An analytical study based on radiographic and clinical follow-up examinations. Acta Odontol Scand. 1956;14:1–175. [Google Scholar]
- 32.Orstavik D, Kerekes K, Eriksen HM. The periapical index: A scoring system for radiographic assessment of apical periodontitis. Endod Dent Traumatol. 1986;2:20–34. doi: 10.1111/j.1600-9657.1986.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 33.Rajendran N, Sundaresan B. Efficacy of ultrasound and color power Doppler as a monitoring tool in the healing of endodontic periapical lesions. J Endod. 2007;33:181–6. doi: 10.1016/j.joen.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Chong BS. Managing Endodontic Failure. Chicago: Quintessence Pub; 2004. [Google Scholar]


