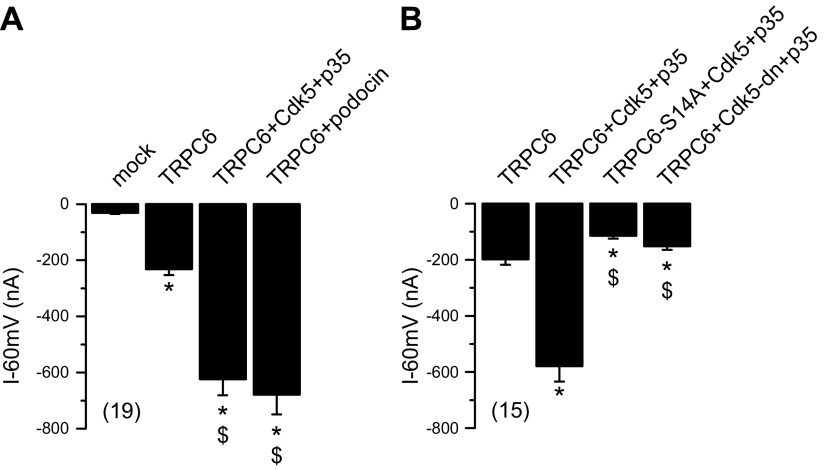
Figure 4.
Phosphorylation of TRPC6 at serine 14 modulates channel activity. TRPC6 channel activity was assessed in double-electrode voltage-clamp experiments in Xenopus oocytes after stimulation with 10 µM OAG. Coexpression of Cdk5 WT and p35 leads to enhanced conductance of TRPC6 channel. A) Summary of whole-cell currents measured at clamp voltage Vc = −60 mV. TRPC6 induced inward current that was augmented in presence of Cdk5/p35, similar to coexpression with podocin. OAG had no effect on mock-injected oocytes. B) Summaries of effects of coexpression with phospho-deficient mutant TRPC6-S14A or Cdk5-dn. Data are presented as means ± sem; values in parentheses indicate number of experiments. *P < 0.05 vs. mock vector (A) or TRPC6 (B) injection (ANOVA); $P < 0.05 vs. TRPC6 (A) or TRPC6 + Cdk5 + p35 (B) (ANOVA).