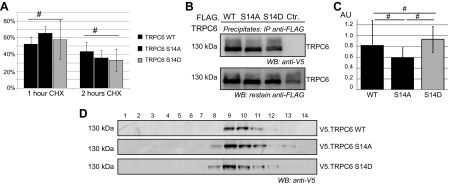
Figure 5.
Phosphorylation at serine 14 does not alter protein stability or oligomerization characteristics of TRPC6. A) HEK 293T cells were transiently transfected with V5-tagged constructs as indicated and treated with 25 µg/ml cycloheximide for time indicated. Band density was quantified after immunoblot analysis of cell lysates with V5-specific antibody and normalized to untreated control. No significant difference in protein stability was detected for TRPC6 WT and phospho mutants (n = 3; Student’s t test). B) Multimerization of TRPC6 was assessed in coimmunoprecipitation experiments. FLAG- and V5-tagged TRPC6 cDNA constructs were transiently expressed in HEK 293T cells as indicated. After immunoprecipitation with FLAG-specific antibody, immunoblotting was performed with V5-specific antibody and restaining of precipitates with FLAG-specific antibody. C) Densitometry quantification of V5 immunoblot shows no significant difference in multimerization for TRPC6 WT and phospho mutants (AU, arbitrary units; n = 3; Student’s t test). D) To investigate complex size of native TRPC6 multimers, HEK 293T cells were transiently transfected with V5-tagged TRPC6 cDNA constructs as indicated, and cell lysates were subjected to velocity gradient centrifugation. Analysis of fractions on immunoblot using V5-specific antibody shows similar sedimentation pattern of TRPC6 WT and TRPC6 S14A and S14D (n = 3). #P > 0.05 (not significant).