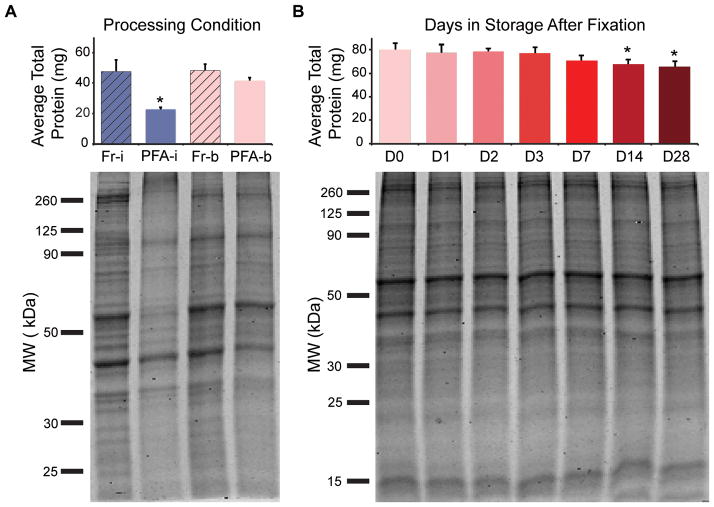
Figure 1. Protein extraction amount and quality are similar between fresh and formaldehyde-fixed/stored samples when using appropriate cell lysis buffer and exposure to high heat.
(A) Total protein amount isolated from fresh (Fr) and PFA-fixed (PFA) ASCs was compared under two lysis conditions (i, on ice; b, by boiling). Data represented as mean ± standard deviation (s.d.) from three, independent experiments. A Kruskal-Wallis test with post-hoc Tukey test was performed to determine significance (* significantly different from unmarked groups, P < 0.05). Coomassie Blue-stained PAGE gels indicated protein quality through comparison of banding profiles. Low protein yields and increased aggregation of proteins (>260 KDa) in the “PFA-i” condition underscore importance of lysis process used to isolate proteins from fixed samples. (B) Total isolated protein was compared across PFA-fixed ASCs stored for 0–28 days. Data represented as mean ± s.d. from one independent experiment run in biological triplicate. A one-way ANOVA with Dunnett’s test (D0 as the point of comparison) was performed to determine significance (* significantly different from D0, P < 0.02). Protein quality assessed by Coomassie Blue-stained PAGE gels.