Abstract
Objective. Immune dysregulation associated with chronic autoimmune diseases, such as SLE, has been associated with increased cancer risk. It is unclear whether isolated cutaneous lupus erythematosus (CLE) modifies cancer risk. We estimated the cumulative incidence of cancer in a population-based CLE cohort and compared the risk with a matched non-CLE cohort.
Methods. All incident cases of CLE in Olmsted County, MN, USA between 1965 and 2005 were identified and followed to December 2013. Estimates for the cumulative incidence of any cancer and skin cancer in patients with CLE were derived and compared with an age-, sex- and calendar-year-matched non-CLE cohort using Cox models.
Results. There were a total of 155 patients with CLE [age at diagnosis, 48 (s.d. 16) years; 65% females; BMI, 26.3 (s.d. 7.1) kg/m2; 40% smokers, 9% with diabetes]. During a median follow-up of 14.6 years, we observed 35 cases of incident cancer (including 10 cases of skin cancer). The cumulative 1-, 5- and 10-year incidence of any cancer after diagnosis of CLE was 1.4, 7.5 and 11.6%, respectively. Compared with matched non-CLE controls, the overall risk of malignancies was not increased in patients with CLE (smoking-adjusted hazard ratio = 1.29; 95% CI: 0.78, 2.13; P = 0.31). There was also no significant increase in risk of any skin cancer in patients with CLE (hazard ratio = 2.51; 95% CI: 0.91, 6.96; P = 0.16).
Conclusion. CLE is not associated with an increased risk of any cancers, including skin cancers, compared with the general population. However, the number of events was small, limiting the power of the study.
Keywords: inflammation, cutaneous lupus, cancer, melanoma, incidence
Rheumatology key messages
-
Cutaneous lupus erythematosus is not associated with an increase in cancer risk in a population-based cohort.
Routine cancer screening practices should suffice for patients with cutaneous lupus erythematosus, without the need for targeted surveillance.
Introduction
Epidemiological studies have shown that chronic inflammatory diseases, such as RA and SLE, are associated with increased risk of haematological, cutaneous and solid-organ malignancies [1, 2]. Even limited autoimmune dermatological conditions, such as psoriasis, are associated with increased risk of both cutaneous and systemic solid-organ malignancies [3]. The increased risk of malignancies in these patients may be attributed to systemic immune dysregulation leading to impairment of tumour surveillance [4] or, alternatively, to immunosuppressive therapy used to treat these autoimmune conditions, which may modify cancer risk [5, 6].
Cutaneous lupus erythematosus (CLE), defined as isolated cutaneous lupus lesions occurring in the absence of significant evidence of SLE, has a 2- to 3-fold higher prevalence than SLE, with a peak incidence in the seventh decade of life [7]. There are limited data on whether CLE affects cancer risk. As the pathogenesis of CLE is similar to SLE, with complex gene–environment interactions, autoimmunity and immune-mediated cutaneous damage, it is conceivable that patients with CLE may be at increased risk for cancer [8]. At the same time, the limited extent of organ involvement with absence of systemic damage suggests that chronic systemic inflammation may be absent in patients with CLE [9].
A Swedish nationwide registry-based study of 3663 patients with CLE followed for 4 years reported a significant increase in the risk of all cancers, buccal cancer, respiratory cancer, lymphoma and non-melanoma skin cancer (NMSC), compared with non-CLE controls [10]. However, the analysis was based on an administrative database, which was not validated for CLE diagnosis, and there was potential misclassification with SLE. Additionally, the database did not capture data on significant confounders, such as smoking.
We undertook this population-based cohort study of patients with CLE in Olmsted County, MN, USA to estimate the cumulative incidence of all cancers, and specifically skin cancers, in patients with CLE and to assess the relative risk of cancers in patients with CLE, compared with age-, sex- and calendar year-matched subjects without CLE.
Methods
This study was approved by the Mayo Clinic and Olmsted Medical Centre Institutional Review Boards. According to institutional policies, all patients had active authorization for use of their medical records for research.
Setting
Olmsted County, situated in Southeastern Minnesota, has a population of 144 260 inhabitants as of the 2010 US Census. Eighty-three per cent of the population is non-Hispanic white, and a substantial proportion is of North European ancestry. The majority of people reside in Rochester, which is the urban centre of an otherwise rural county. The residents of Olmsted County are socio-economically comparable to the US white population, although a higher proportion are employed in health-care services (25% in Olmsted County vs 8% nationwide) and have a higher level of education (30% completed college in Olmsted County vs 21% nationwide) [11, 12].
Medical care within Olmsted County, MN is practically self-contained within the region and is provided by Mayo Clinic, Olmsted Medical Centre and their affiliated hospitals, and the Rochester Family Medicine Clinic [13]. These health-care providers are connected through a unique medical records-linkage system [the Rochester Epidemiology Project (REP)], which allows for the conduct of true population-based research studies. Each year, >80% of the Olmsted County population is examined by one of the providers participating in the REP, and during any given 3-year period, >90% of local residents are examined at one of the REP providers.
The linkage of information essentially enumerates the Olmsted County population; in fact, REP population estimates are consistently higher than those reported by the US Census [14]. The central diagnostic index of REP comprises all diagnoses generated from outpatient evaluations, hospitalizations, emergency room evaluations, nursing home visits, surgical procedures, autopsy reports and death certificates. It is therefore possible to identify all cases of a disease for which patients sought medical attention over a particular period of time.
CLE case definition
Cases with isolated cutaneous lupus were identified using International Classification of Diseases-9 (ICD-9) codes. We used the REP linkage system to identify all medical records for residents of Olmsted County who had an established diagnosis of any of the subtypes of CLE from 1 January 1965 to 31 December 2005, including classic discoid lupus erythematosus, lupus panniculitis, bullous lupus erythematosus and subcutaneous lupus erythematosus confirmed by dermatological evaluation and histopathological analysis. Data were collected regarding patient demographics, serology, biochemical markers of inflammation (ESR, CRP) and treatment (details of serological tests are given in the supplementary Data, available at Rheumatology Online). We excluded non-residents of Olmsted County and patients with CLE who also had SLE at the time of diagnosis. Cases of drug-induced CLE were also excluded.
Using the definition of Sontheimer [15], subacute CLE lesions were described as a photosensitive, non-scarring, non-atrophy-producing, annular, erythematous, papulosquamous or psoriasiform rash. Patients were subclassified as having annular or psoriasiform subacute CLE. Classic discoid lupus erythematosus was defined according to the classification criteria of Gilliam and Sontheimer [15] as the presence of discoid lesions without subacute CLE or SLE. Patients were subclassified as having localized or generalized discoid CLE. Lupus panniculitis was defined as circumscribed subcutaneous nodules. Diagnosis of all forms of CLE was determined by clinical, serological, histopathological and immunopathological findings. The date of diagnosis was determined as the date of fulfilment of criteria for subtype definition.
Control subjects without CLE were identified from the same Olmsted County population and were matched, 1:1, with cases on age (birth year ±3 years), sex and index date of diagnosis of CLE.
Cancer diagnosis
Using the medical records linkage system, all cancers, including haematological malignancies, solid-organ cancers, melanoma and NMSC, in patients with CLE were identified, including those occurring before and after CLE diagnosis, and diagnosed at outpatient clinic visits or hospitalizations. Subsequently, all medical records were manually reviewed to verify the diagnoses.
Statistical analysis
Descriptive statistics (percentages, mean, etc.) were used to summarize the characteristics of each cohort, and comparisons between cohorts were performed using χ2 and rank sum tests. The cumulative incidences of all cancers and skin cancers (including melanoma and NMSC), adjusted for the competing risk of death, were estimated [16]. Patients with cancer prior to CLE incidence/index date were removed from these analyses because they were not at risk of developing their first cancer of that type. Cox proportional hazard models were used to examine the association between CLE/non-CLE and the development of cancer adjusting for age, sex, smoking and calendar year of CLE incidence/index date. Further adjustments were not performed because of limited statistical power. To assess the effect of the extent of cutaneous involvement (as a possible reflection of inflammatory burden) on cancer risk, we compared the risk of cancer in patients with generalized CLE with those of localized discoid CLE.
Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria). Values of P < 0.05 were considered statistically significant.
Results
The study population consisted of 155 patients with CLE and 155 age-, sex- and calendar year-matched individuals without CLE (Table 1). The mean age at CLE diagnosis/index date was 48 (s.d. 16) years, and 65% were females. Of the 155 CLE patients, 88% were Caucasian. The CLE cohort included 128 patients with discoid CLE (73%; 91 with localized and 37 generalized), 23 with subacute CLE (15%; 6 annular and 17 psoriasiform), 3 with lupus panniculitis (2%) and 1 with bullous CLE (1%). Overall, 46 patients (30%) were treated with HCQ, and 6 patients (4%) were treated with prednisone (classified as users if they used medication for at least 3 months). The median (interquartile range) ESR and CRP at CLE diagnosis were 10 (4–20) mm at 1 h (n = 131) and 2.9 (1.5–3.7) mg/l (n = 21), respectively. Autoantibody data were present for a subset of patients, as follows: ANA positive, 70/146 (47.9%); dsDNA positive, 6/105 (5.7%); anti-Smith antibody positive, 1/79 (1.3%); anti-SSA/Ro positive, 32/80 (40.0%); anti-SSB/La positive, 10/79 (12.7%); and anti-RNP positive, 6/78 (7.7%). Total complement levels were abnormal in 7/82 (8.5%). At the time of last follow-up, 3/6 patients positive for anti-dsDNA progressed to SLE, whereas one patient positive for anti-Smith antibody did not progress to SLE.
Table 1.
Baseline characteristics of patients with cutaneous lupus erythematosus and subjects without cutaneous lupus erythematosus
| Characteristic | Patients with CLE (n=155) | Subjects without CLE (n=155) | P-values |
|---|---|---|---|
| Demographics | |||
| Age, mean (s.d.), years | 48 (16) | 48 (16) | 0.96 |
| Sex, female, n (%) | 100 (65) | 100 (65) | 1.00 |
| Follow-up, median (interquartile range), years | 14.6 (8.1–23.6) | 15.8 (8.7–24.3) | – |
| Race, white (%)a | 117 (88.0) | 136 (98.6) | 0.03 |
| History of malignancy prior to index date | |||
| Any cancer, n (%) | 11/155 (7.1) | 14/155 (9.0) | 0.53 |
| Any cancer, n (%)b | 3/155 (1.9) | 5/155 (3.2) | 0.57 |
| Co-morbidities | |||
| Smoking, current, n (%)c | 61 (39.6) | 32/155 (20.6) | 0.001 |
| BMI, mean (s.d.), kg/m2d | 26.3 (7.1) | 26.5 (5.1) | 0.18 |
| Cardiovascular disease, n (%) | 17/155 (11.0) | 11/155 (7.1) | 0.23 |
| Hypertension, n (%)e | 85/155 (54.8) | 72 (46.8) | 0.16 |
| Diabetes mellitus, n (%)f | 24 (15.7) | 18 (12.2) | 0.38 |
| Hyperlipidaemia, n (%)g | 62/155 (40.0) | 72 (47.1) | 0.22 |
| Family history of early CAD, n (%)d | 6 (4.1) | 6 (3.9) | 0.95 |
aData available for only 133 patients with CLE and 138 subjects without CLE.
bAll skin cancers prior to index date were non-melanoma skin cancers; there were no cases of melanoma.
cData available for only 154 patients with CLE.
dData available for 147 patients with CLE and 144 non-CLE controls.
eData available for only 154 subjects without CLE.
fData available for only 153 patients with CLE and 148 subjects without CLE.
gData available for only 153 patients with CLE.
hData available for only 147 patients with CLE and 152 subjects without CLE.
CAD: coronary artery disease; CLE: cutaneous lupus erythematosus.
Eleven patients with CLE (including 3 patients with skin cancer) and 14 patients without CLE (including 5 patients with skin cancer) had a history of malignancy prior to CLE diagnosis/index date. Patients with CLE were more likely to be current smokers compared with the non-CLE cohort (39.6 vs 20.6%, P = 0.001).
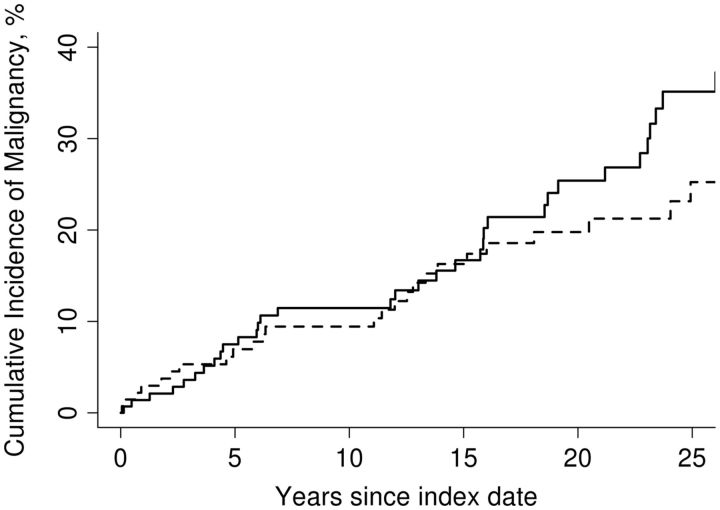
After excluding patients with cancer prior to CLE diagnosis, 35 patients with CLE developed new cancer over a median 14.6 years (interquartile range, 8.1–23.6 years) of follow-up. These included 11 patients with skin cancer (9 patients with NMSC and 2 patients with melanoma), 3 cases of haematological malignancies and 21 cases of solid-organ cancers (including 3 gastrointestinal cancers). The cumulative 10-year incidence of any cancer after diagnosis of CLE was 11.5% (Fig. 1). The risk of any cancer was not significantly different compared with the non-CLE cohort [adjusted hazard ratio (HR) = 1.29; 95% CI: 0.78, 2.13]. After excluding NMSC, the risk of any cancer was also not significantly different in patients with CLE compared with the non-CLE cohort (adjusted HR = 1.07; 95% CI: 0.61, 1.87). The cumulative 10-year incidence of any skin cancer after diagnosis of CLE was 4.3% (supplementary Fig. S1, available at Rheumatology Online). The risk of any skin cancer was not significantly different compared with the non-CLE cohort (adjusted HR = 2.51; 95% CI: 0.91, 6.96). Separate analysis of risk of melanoma was not feasible because of the small number of events.
Fig. 1.
Cumulative incidence of any cancer after diagnosis of cutaneous lupus erythematosus
Continuous lines represent patients with CLE and dashed lines represent matched non-CLE controls. CLE: cutaneous lupus erythematosus.
In order to assess whether the extent of cutaneous involvement (as a marker of inflammatory burden) was associated with cancer risk in patients with CLE, we compared the risk of any cancer in patients with generalized vs localized discoid CLE. There was no significant difference in the risk of cancer in patients with generalized discoid CLE compared with localized discoid CLE (adjusted HR = 1.52; 95% CI: 0.72, 3.19).
Discussion
Chronic inflammatory diseases, such as SLE, are associated with increased risk of cancer, perhaps related to systemic immune dysregulation leading to impairment of tumour surveillance or, alternatively, to immunosuppressive therapy used to treat these conditions. CLE is a skin-limited autoimmune condition, and its treatment usually does not involve as much immunosuppressive therapy as is required for SLE. In our population-based inception cohort of 155 patients with CLE followed over 15 years, we observed that patients with CLE are not at increased risk of cancers, including skin cancers, compared with age-, sex- and calendar year-matched individuals without CLE. However, it is important to acknowledge that the number of events was small, limiting the power of the study.
Our findings are in contrast to observations from a Swedish nationwide cohort study. In their study of 3663 patients with CLE, Grönhagen and colleagues [10] identified 183 cases of cancer over a median follow-up of 4.1 years, with a significant increase in the risk of all cancers compared with matched controls without CLE (HR = 1.8; 95% CI: 1.5, 2.2). The risk for buccal cancer, respiratory cancer, lymphoma and NMSC was increased in this cohort, without a significant increase in risk of melanoma, haematopoetic and other forms of cancer. However, their case definition (single ICD-10 code for CLE) was non-validated, with potential for misclassification; 35% of patients in their CLE cohort were diagnosed with SLE (received a diagnostic code) either before (23%) or after (12%) CLE diagnosis. When patients with SLE were excluded, an increased risk of cancer persisted. Additionally, about 24% of these ‘incident’ cancers were diagnosed within 1 year of CLE, suggestive of diagnostic suspicion bias around the time when CLE was diagnosed. This is particularly true of NMSCs, which are diagnosed on a thorough skin examination (to which patients with CLE may be subjected). The highest relative risk was observed with buccal and respiratory cancer, both of which are smoking related; however, they were unable to adjust for smoking in their analysis. We, and others, have observed that smoking is more prevalent in patients with CLE compared with controls [17].
Our study has several strengths, which support the validity of our findings. Ours is a well-characterized population-based inception cohort of patients with CLE from a defined geographical area with a stable population, over an extended period of time. Our medical records linkage system allowed confirmation of CLE as well as cancer diagnosis through chart review, rather than relying solely on administrative diagnostic codes, minimizing the risk of misclassification. We carefully excluded patients with concomitant diagnosis of SLE. Finally, by comparing cancer risk in patients with generalized vs localized discoid CLE (the most common form of CLE in our cohort), we were able to estimate whether the extent of cutaneous involvement, as a possible surrogate for degree of inflammation, may influence cancer risk.
Limitations of the study include the small sample size; hence, the limited power for some detailed comparative analyses. The population of Olmsted County is predominantly Caucasian (90.3% at US 2000 census), which may limit the generalizability of the findings to other ethnicities. With increasing racial diversity in Olmsted County, it will be crucial to examine future trends. Owing to the retrospective nature of this study, we were unable to assess CLE disease activity and therapy comprehensively.
In conclusion, our findings suggest that patients with CLE are not at increased risk of cancers, including skin cancers, compared with matched controls. However, the number of events was small, limiting power of the study. Routine cancer screening practices, as in the general population, may suffice for patients with CLE, without the need for targeted surveillance.
Supplementary Material
Acknowledgements
Author Contributions: Study concept and design: A.G.S., C.S.C., H.M.K., E.L.M. and V.R.C. Acquisition of data: A.G.S. Analysis and interpretation of data: A.G.S., C.S.C., H.M.K., E.L.M. and V.R.C. Drafting of the manuscript: A.G.S., S.S. Critical revision of the manuscript for important intellectual content: C.S.C., M.P.D., H.M.K., E.L.M. and V.R.C. Approval of the final manuscript: A.G.S., C.S.C., M.D.P.D., S.S., H.M.K., E.L.M., V.R.C. Guarantor of the Article: V.R.C.
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Cao L, Tong H, Xu G. et al. Systemic lupus erythematous and malignancy risk: a meta-analysis. PLoS One 2015;10:e0122964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pouplard C, Brenaut E, Horreau C. et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol 2013;27 (Suppl 3):36–46. [DOI] [PubMed] [Google Scholar]
- 4. Chow MT, Möller A, Smyth MJ. Inflammation and immune surveillance in cancer. Semin Cancer Biol 2012;22:23–32. [DOI] [PubMed] [Google Scholar]
- 5. Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA. et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. JAMA 2012;308:898–908. [DOI] [PubMed] [Google Scholar]
- 6. Yadav S, Singh S, Harmsen WS. et al. Effect of medications on risk of cancer in patients with inflammatory bowel diseases: a population-based cohort study from Olmsted County, Minnesota. Mayo Clin Proc 2015;90:738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jarukitsopa S, Hoganson DD, Crowson CS. et al. Epidemiology of systemic lupus erythematosus and cutaneous lupus erythematosus in a predominantly white population in the United States. Arthritis Care Res 2015;67:817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin JH, Dutz JP, Sontheimer RD, Werth VP. Pathophysiology of cutaneous lupus erythematosus. Clin Rev Allergy Immunol 2007;33:85–106. [DOI] [PubMed] [Google Scholar]
- 9. Werth VP. Clinical manifestations of cutaneous lupus erythematosus. Autoimmun Rev 2005;4:296–302. [DOI] [PubMed] [Google Scholar]
- 10. Grönhagen CM, Fored CM, Granath F, Nyberg F. Increased risk of cancer among 3663 patients with cutaneous lupus erythematosus: a Swedish nationwide cohort study. Br J Dermatol 2012;166:1053–9. [DOI] [PubMed] [Google Scholar]
- 11. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. United States Census Bureau. United States Census Bureau Quick Facts. United States Census Bureau, Vol. 2014; 2014. [Google Scholar]
- 13. St Sauver JL, Grossardt BR, Yawn BP. et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 2011;173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol 1981;4:471–5. [DOI] [PubMed] [Google Scholar]
- 16. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- 17. Koskenmies S, Järvinen TM, Onkamo P. et al. Clinical and laboratory characteristics of Finnish lupus erythematosus patients with cutaneous manifestations. Lupus 2008;17:337–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.