Abstract
Patients with HF and their families experience stress and suffering from a variety of sources over the course of the HF experience. Palliative care is an interdisciplinary service and an overall approach to care that improves quality of life and alleviates suffering for those living with serious illness, regardless of prognosis. In this review, we synthesize the evidence from randomized clinical trials of palliative care interventions in HF. While the evidence base for palliative care in HF is promising, it is still in its infancy and requires additional high-quality, methodologically sound studies to clearly elucidate the role of palliative care for patients and families living with the burdens of HF. Yet, an increase in attention to primary palliative care (e.g., basic physical and emotional symptom management, advance care planning), provided by primary care and cardiology clinicians, may be a vehicle to address unmet palliative needs earlier and throughout the illness course.
Keywords: Palliative care, hospice, heart failure, quality of care, quality of life
Introduction
Heart failure (HF) is a chronic, progressive, and ultimately lethal disease that affects >6 million American adults, with an additional 870,000 individuals diagnosed annually (1). Despite advances in HF therapies, nearly 40% of patients will die within a year of their first hospitalization (2). During the course of HF, patients typically experience debilitating physical and emotional symptoms, loss of independence, and disruptions to social roles, all of which severely degrade quality of life (QoL) (3,4). Physical symptoms in advanced HF, such as pain, are highly distressing for patients and caregivers, yet remain under-recognized and undertreated (5,6). Patients and their caregivers (7,8) often face decisions about high-risk and complex treatments (e.g., cardiac devices, transplantation) without adequate prognosis communication, decision support, or advance care planning (9,10). In addition, HF management poses enormous financial and resource stress on families, healthcare systems, and society; direct medical costs of HF are projected to be >$77 billion by 2030, a 215% increase from current spending (11).
Palliative care is an interdisciplinary approach, as well as a clinical subspecialty that focuses on improving QoL and reducing suffering among patients with serious illness and their families (12). Core domains of palliative care interventions include: expert assessment of pain and other physical symptoms, psychosocial care, identification of goals of care, and support for complex treatment and decision making. A recent systematic review and meta-analysis of palliative care interventions suggests that a palliative approach is associated with improved patient QoL, reduced symptom burden, and improved caregiver outcomes (13). However, most evidence for palliative care emanates from oncology; the role of palliative care in chronic, non-malignant illnesses such as HF is underdeveloped (13).
Palliative care takes many forms. Historically, a sub-specialty trained palliative care specialist works alongside patients’ primary clinicians to consult on or co-manage patients’ palliative needs. Alternatively, primary palliative care (or “basic” or “generalist” palliative care) is the concept that all clinicians, regardless of specialization, should be competent in fundamental palliative skills (14). These skills include basic physical and emotional symptom management, initial goals of care discussions, and patient referral to specialty palliative care or, for patients at the end of life, hospice care. Palliative care also varies by the location of service. More than 65% of U.S. hospitals have a specialty palliative care program which delivers services to inpatients (15). Community- and outpatient-based palliative care models have been regarded as the “new frontier” in supporting patients and families longitudinally and across a variety of care settings (16).
In this review, we describe the potential role of palliative care in improving outcomes in patients with HF, characterize typical palliative care delivery models and each model’s existing evidence, and describe future priorities for palliative care research and clinical practice models in HF.
NATURAL OPPORTUNITIES TO INTEGRATE PALLIATIVE DOMAINS IN HF CARE
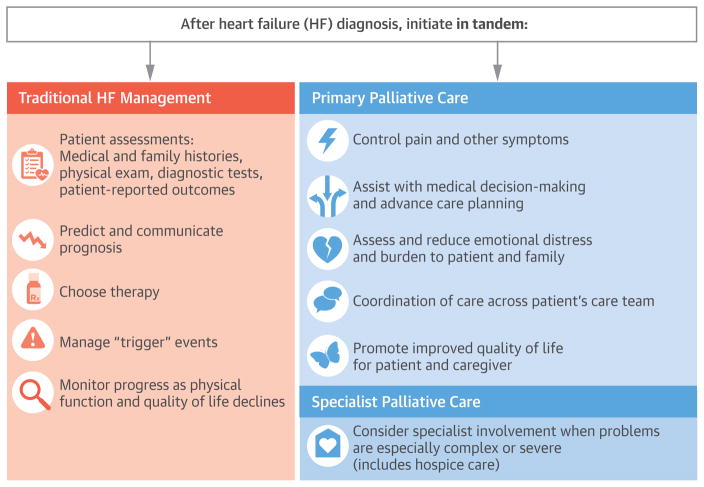
Historically, the prevailing approach to palliative care has been one of a zero-sum game; palliative and curative therapies have been erroneously regarded as contradictory options(17). It is no longer appropriate to assume that palliative care should be initiated only as a treatment of last resort when traditional HF management fails to fulfill a patient’s goals. Particularly given the unpredictable trajectory of HF, waiting for a “trigger” event at which to initiate a palliative approach – either primary palliative care or specialty palliative care consultation – perpetuates the false dichotomy of palliative versus (rather than palliative plus) life-prolonging therapy. In fact, there are often multiple natural opportunities to consider integrating various palliative domains throughout the HF trajectory (Central Illustration). For many patients, primary palliative care, such as basic symptom management and identifying a surrogate decision maker is provided by a primary care, cardiology, or HF clinician. Palliative care specialists can assist with the management of intractable symptoms, and more complex medical decision-making, such as instances of discordant patient-family goals or irresolvable unrealistic expectations of medical therapies. Recognizing the potential discordance between objective measures of disease severity (e.g., ejection fraction) and patient-reported outcomes (e.g., symptom burden, QoL), it is important that patient-reported outcomes, such as symptoms and QoL be monitored regularly throughout the entire HF experience by primary care and/or cardiology providers, so as to facilitate optimal patient-centered care. Ultimately, the optimal timing for integrating primary or specialty palliative care for patients with advanced HF will vary, reflecting patient need, not prognosis.
Central Illustration. Integrating Palliative Care Across the Heart Failure Experience.
Core domains of primary palliative care (e.g., symptom assessment and management, psychosocial support, advance care planning) may be seamlessly integrated within usual HF disease and device management. When appropriate, specialty palliative care services may be initiated to address complex or intractable palliative needs. The timing of these referrals should be based on patient need, not prognosis, and can be initiated at any point during the HF trajectory. Stars depict key events, such as acute decompensation or hospitalization, which may be particularly salient opportunities for evaluation of appropriateness for specialty palliative care referral or hospice referral, if aligned with a patient’s goals. Given that symptoms, functional status, and QoL are not perfectly correlated, it is important that palliative needs such as symptoms and QoL be routinely and systematically monitored throughout the patient’s HF care trajectory.
Poorly controlled symptoms and psychosocial-spiritual distress
Patients with HF often have a wide array of symptoms, including dyspnea, pain, anxiety, depression, sleep disturbance, and fatigue (18,19). There are varying levels of evidence for treating HF symptoms (20). Often, the ideal HF symptom management approach is treating the underlying HF condition (e.g., relieving dyspnea by addressing fluid overload); this is a clear example of the harmony between traditional HF disease management and a palliative approach (21). However, many symptoms persist despite optimal disease management. For example, pain is common, yet under-recognized and therefore undertreated in HF (5). Similarly, depression occurs in an estimated 1 in 5 patients with HF, and is associated with worse QoL and increased mortality(22); yet routine screening for depression in HF is rare (23,24).
The psychosocial-spiritual context of HF beyond depression and anxiety is understudied (25). The HF experience is rife with uncertainty, existential distress, and adjustment to modified social and professional roles. Additionally, patients considering advanced therapies such as VADs and cardiac transplantation face additional anxieties as they anticipate or adjusting to a new life post-receipt. In addition to limitations in personal roles, patients experience wide variability in social support and the availability of informal caregivers (e.g., friends, spouses, children) (26,27). Regarding spirituality, patients with HF and poor health status report worse spiritual well-being compared to patients with metastatic lung and pancreatic cancer (28).
The assessment and treatment of physical symptoms and psychosocial-spiritual distress in HF should be the responsibility of patients’ ongoing care providers (i.e., primary care, cardiology, mental health) and community supports. Yet the assessment and treatment of HF symptoms need not wait until the point of intractability; palliative care principles should be integrated throughout the HF management continuum, allowing cardiology and primary care clinicians to serve as primary palliative care providers, alleviating symptoms before they become overly burdensome. Although the role of palliative care specialists is still being defined, if patient distress persists and palliative care specialists are available, referral should be considered.
Hospitalization and Discharge
Patients with HF have a higher rate of acute care service utilization in the 30 days before death than patients with cancer (ED visits: 64% vs. 39%, hospitalizations: 60% vs. 45%, and ICU admissions: 19% vs. 7%) (29–34). Each hospital admission is an opportunity to discuss goals of care, as this is most likely when the treatment regimen for a patient with HF may escalate. As the risk for mortality increases with each subsequent hospitalization, hospital discharge planning is an opportunity to discuss what is most important, what QoL means to the patient/family, and under what circumstances they would and would not want life prolonging treatments (35). Furthermore, depending on the treatments initiated, the patient may require further assistance on discharge, such as home care, physical therapy, or cardiac rehabilitation. In addition, many families are intimately involved in patient care. Caregivers suffer physical, psychological and financial consequences associated with this care (27). Therefore, clinicians should screen for caregiver burden and stress and help by providing support and counselling.
End-of-Life Transition
Although commonly conflated, palliative care and hospice are related but conceptually distinct services (17). Palliative care is both a clinical specialty, and an overall approach to care that focuses on improving QoL and relieving suffering for patients and families facing serious illness, based on need and not prognosis. Hospice care is a specific delivery mechanism of palliative care reserved for individuals at the end of life. In contrast to palliative care, hospice eligibility (in the U.S.) requires an estimated life expectancy of six months or less, and an agreement to forego life-sustaining procedures. One exception is the U.S. Veterans Health Administration, which allows for hospice care concurrent with life-sustaining treatments. Addressing a patient’s physical, psychosocial, and existential distress need not wait until the very end of life; palliative care should be seamlessly integrated throughout the HF experience, with referral to hospice services if and when its philosophy aligns with patient and family goals.
Patients with advanced HF (i.e., ACC/AHA stage D) have an estimated 1-year mortality of 29%, and an estimated 1-year freedom from hospitalization or death of only 32.9%(36). Despite this high morbidity and mortality, hospice utilization has remained low with about one-third of patients with HF receiving hospice at time of death (37). Patients with advanced HF enroll in hospice at lower rates than those with cancer (19) and compared to patients with cancer, patients with HF were more likely to enroll in hospice late in the course of their disease (within three days of death)(38). Nevertheless, numerous cardiology professional societies have called for the continued and earlier integration of hospice care for patients with advanced heart disease(39–43). Further training is needed to assist primary care and HF clinicians to identify patients who are eligible for hospice, to describe what hospice care can provide in different settings (i.e. home, inpatient and residence), and to introduce hospice as a treatment recommendation when appropriate. When conflict arises between patients and/or families or between clinicians about a hospice recommendation, specialty palliative care may be helpful in facilitating future treatment care planning.
REVIEW OF RANDOMIZED CLINICAL TRIALS OF PALLIATIVE CARE IN HEART FAILURE
Using a recently published systematic review of randomized clinical trials of palliative care interventions (13), we conducted a secondary analysis of studies that either exclusively enrolled patients with HF or reported results separately by disease group. Briefly, we searched MEDLINE, EMBASE CINAHL, and Cochrane Library’s CENTRAL, from database inception to July 22, 2016. Randomized clinical trials (RCTs) were eligible for inclusion if their interventions comprised at least two of the eight domains included in the definition of palliative care from the National Consensus Project for Quality Palliative Care (44). Two investigators independently screened and reviewed the resulting 6,158 unique records, ultimately yielding 43 trials; of these 43, five trials either only included patients with HF or presented data by disease group, and were therefore eligible for inclusion. One relevant additional trial was hand selected as it was published after our initial search (45). Each study was evaluated for risk of bias for subjective outcomes (e.g., patient-reported outcomes) and objective outcomes (e.g., survival, resource utilization) using the Cochrane Risk of Bias tool (46). A complete description of the search and analytic methodology is available elsewhere (13). Although this search is restricted to six studies of the strongest methodological design (i.e., RCTs), it should be noted that some quasi-experimental and observational studies have demonstrated potential benefits of palliative care interventions in HF patients (47–50).
Inpatient Specialty Consultation or Co-management
Two RCTs of inpatient specialty team-based consultation yielded mixed results about the impact of palliative care on healthcare utilization, yet provide some evidence for potential benefits of palliative care on patient-level outcomes (Table 1). However, because both trials were deemed to be at high risk of bias results should be interpreted cautiously.
Table 1.
Summary of Existing Trials of Inpatient Specialty Palliative Care in Heart Failure
| Study (Country) | Patient Population* | Intervention (Participants Randomized) | Control (Participants Randomized) | Results | Risk of Bias | |
|---|---|---|---|---|---|---|
| Subjective | Objective | |||||
| Hopp et al, 2016 (USA) | Acute HF, 1-yr mortality risk of ≥33%, and/or NYHA Class III–IV (Mean age: 68) | Inpatient specialist consultation from a multidisciplinary team (physician, nurse practitioner, chaplain, social worker) conducted clinical interview(s), assessing symptoms, goals of care and post- treatment location desires, and advance care planning (n=43) | Usual care (n=42) | Hospice utilization/ACP (Composite outcome): NS; difference between groups 9.3% (95% CI: −11.8%, 30.0%) | High | High |
| Sidebottom et al, 2014 (USA) | Acute HF (Mean age: 73) | Specialty multidisciplinary palliative care consultation assessing physical and emotional symptoms, spiritual, and social aspects of care. (n=116) | Usual care (n=116) |
QOL [Minnesota Living with Heart Failure Questionnaire]: Improved, mean difference 3.06 points (95% CI: 2.75, 3.37) Symptom burden [ESAS]: Improved total symptom burden, mean difference 4.31 points (95% CI: 4.00, 4.62) Six-month mortality: NS; HR, 1.90 (95% CI: 0.88, 4.09) 30-day hospital readmission: NS; HR, 1.43 (95% CI: 0.5, 4.1) Hospice use within 6 months: NS; HR, 1.60 (95% CI: 0.58, 4.38) ACP within 6 months: Improved; HR, 2.87 (95% CI: 1.09, 7.59) Mood [PHQ-9]: Improved; mean difference, 0.72 (95% CI: 0.41, 1.03) |
High | High |
ACP=Advance Care Planning. CI=Confidence Interval. ESAS= Edmonton Symptom Assessment Scale. HF=Heart Failure. NS=Not significant. NYHA=New York Heart Association. PHQ-9= Patient Health Questionnaire 9. QOL=Quality of Life. USA=United States of America.
Patient population details the indication for palliative care. All comparisons stated as intervention vs. control.
A 2015 trial compared the impact of inpatient consultation by a palliative care team versus usual care for patients hospitalized for acute HF(51). The authors reported statistically significant improvements for all patient-reported outcomes measured, including QoL, symptom burden, and mood. There was no effect on patient survival. Although the intervention was associated with increased advance care planning, no effect was found regarding 30-day hospital readmission, nor on hospice referral. Strengths of this study included the use of a multi-professional team approach similar to the ideal model of palliative care delivery in inpatient settings (i.e. palliative care physicians, advance practice nurses, social workers, and chaplains). However, given that patients in the trial were financially responsible for any subsequent palliative care visits, the majority of patients (80%) received only one visit which does not allow comment on the effects of a more longitudinal palliative approach.
Hopp and colleagues evaluated the effect of inpatient palliative care consultation within three urban U.S. hospitals (n=85). Intervention content included symptom assessment and management, elicitation of goals of care, advance care planning, and discharge planning (52). No effect was found regarding the trial’s composite primary outcome at 3–6-month follow-up of hospice utilization or the creation of a “do not resuscitate” order during or after the index hospitalization (difference, 9.3%), but with wide confidence intervals (95% CI −11.8% to 30.0%; p = 0.12). No statistically significant effect was found regarding patient survival (p=0.47).
Outpatient Specialty Palliative Care
The most compelling evidence of the benefits of palliative care in HF arguably comes from the recently published PAL-HF (Palliative Care in Heart Failure) study (low risk of bias; Table 2) (45). This trial randomized 150 recently hospitalized individuals with advanced HF at high risk of re-hospitalization or six-month mortality to either usual care or usual care plus a six-month interdisciplinary palliative care intervention led by a palliative care-specialized nurse practitioner. The protocolized intervention aimed to improve patient QoL by addressing physical and emotional symptoms, spiritual concerns, and advance care planning. Compared to usual care, the palliative care intervention was associated with clinically significant improvements in HF-specific and disease-generic QoL at six-month follow-up (mean difference on KCCQ, 9.49 points; 95% CI: 0.94, 18.05; mean difference on FACIT-Pal, 11.77 points; 95% CI: 0.84, 22.71). The trial also reported statistically significant improvements in secondary outcomes such as mood, and spiritual wellbeing. The intervention was not found to be associated with mortality or re-hospitalization.
Table 2.
Summary of Existing Trials of Outpatient Specialty Palliative Care in Heart Failure
| Study (Country) | Patient Population* | Intervention (Participants Randomized) | Control (Participants Randomized) | Results | Risk of Bias | |
|---|---|---|---|---|---|---|
| Subjective | Objective | |||||
| Rogers et al, 2017 (USA) | Hospitalization for HF in past year and ESCAPE score >=4 indicating >50% risk of 6-mo. mortality. (Mean age: 71) | Interdisciplinary NP-led specialty palliative care intervention concomitant with usual HF management. Intervention foci included: physical and emotional symptom management, spiritual concerns, and advance care planning. (n=75) | Usual care (n=75) |
QOL [KCCQ]: Improved at 6 months, mean difference 9.49 points (95% CI: 0.94, 18.05; p=0.03) [FACIT-Pal]: Improved at 6 months, mean difference 11.77 points (95% CI: 0.84, 22.71; p=0.035) Mood [HADS depression]: Improved at 6 months, mean difference −1.94 points (95% CI: 3.57, −0.31; p=0.02) [HADS anxiety]: Improved at 6 months, mean difference −1.83 points (95% CI: −3.64, −0.02; p=0.048) Spiritual wellbeing [FACIT-Sp]: Improved @ 6 months, mean difference 3.98 points (95% CI: 0.46, 7.50; p=0.027) 6-month mortality: NS, 30.7% vs 26.7% (p value not reported). HF-related Rehospitalization: NS, 30.7% vs. 29.3% (p value not reported) |
Low | Low |
CI=Confidence Interval. ESCAPE=Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness. FACIT-Pal=Functional Assessment of Chronic Illness Therapy - Palliative care. FACIT-Sp=Functional Assessment of Chronic Illness Therapy - Spiritual Well-Being. HADS= Hospital Anxiety and Depression Scale. HF=Heart Failure. KCCQ=Kansas City Cardiomyopathy Questionnaire. NP=Nurse Practitioner. NS=Not significant. QOL=Quality of Life. USA=United States of America.
Patient population details the indication for palliative care. All comparisons stated as intervention vs. control.
Home-Based Specialty Palliative Care
Two RCTs of home-based palliative care interventions enrolled individuals with advanced disease (NYHA class III–IV) and provided palliative content embedded within a larger framework of disease management, including care coordination and a multidisciplinary team approach (Table 3) (53,54). Although results are generally promising, both trials were deemed to be at high risk of bias; therefore, findings should be cautiously interpreted (13).
Table 3.
Summary of Existing Trials of Home-Based Specialty Palliative Care in Heart Failure
| Study (Country) | Patient Population* | Intervention (Participants Randomized) | Control (Participants Randomized) | Results | Risk of Bias | |
|---|---|---|---|---|---|---|
| Subjective | Objective | |||||
| Brännström et al, 2014 (Sweden) | NYHA Class III–IV HF (Mean age: 79) | Predominately in-home HF disease management and palliative care services via a multidisciplinary approach and care coordination (n=36) | Usual care (n=36) |
QOL [EQ5D]: Improved; (57.6 ± 19.2 vs. 48.5 ± 24.4; P=0.05) [KCCQ]: NS (data not reported) Symptom burden [ESAS]: NS (data not reported) Six-month survival: NS; P=0.34 Hospitalizations: reduced; mean (SD)=0.42 (0.60) vs. 1.47 (1.81); P=0.009 Total costs: NS; mean €4078 vs. €5727 (P not reported) Increased proportion of patients with improved NYHA class (39% vs. 9%; P=0.015) |
High | High |
| Wong et al, 2016 (Hong Kong) | Advanced HF (e.g. NYHA stage III–IV) (Mean age: 78) | Palliative care home nurses conducted home visits/telephone calls providing transitional palliative care (n=43) | Usual care (n=41) |
Symptom burden [ESAS]: Proportion of patients with improvement in total score, 73% vs. 41.4%, P<0.05 QOL [McGill]: Improved at 4 weeks; 7.57 points vs. 6.46 points; P<0.001 [Chronic HF Questionnaire]: Improved at 4 weeks; 5.26 points vs. 4.47 points; P<0.001 Satisfaction with care: Higher at 4 weeks; 48.84 points vs. 3.55 points, P<0.001 Hospital readmission: NS at 4 weeks; 20.9% vs. 29.3%, P=0.38; Reduced at 12 weeks: 33.6% vs. 61%, P=0.009 |
High | High |
ACP=Advance Care Planning. ESAS= Edmonton Symptom Assessment Scale. EQ5D= EuroQol Five Dimensions Questionnaire. HF=Heart Failure. KCCQ= Kansas City Cardiomyopathy Questionnaire. NS=Not significant. NYHA=New York Heart Association. QOL=Quality of Life. SD=Standard deviation. USA=United States of America.
Patient population details the indication for palliative care. All comparisons stated as intervention vs. control.
Brännström and colleagues conducted a trial of a home-based integrated HF disease management and palliative care intervention delivered by a multidisciplinary team (54). Compared to usual care, patients who received the palliative care intervention reported statistically significant improvements in QoL at 6 weeks, despite no effect on symptom burden. The intervention was associated with fewer hospitalizations over six months (mean 0.42 vs 1.47, p = 0.009), while there was no association identified regarding total costs of care. In addition, a greater proportion of patients in the intervention group experienced improvement in NYHA functional class at 6 months (39% vs. 9%, p = 0.015).
In a trial of transitional palliative care, Wong and colleagues randomized 84 patients recently discharged from the hospital to receive a combination of home visits and telephone check-ins from palliative care home nurses, or an attention control (i.e., social phone calls regarding unrelated topics) (53). At 12-week follow-up, intervention patients had significantly fewer hospital readmissions than control patients (relative risk (95% CI), 0.55 (0.35, 0.88)), and higher satisfaction with their healthcare. At 12 weeks, the intervention was also associated with reduced symptom burden, and improved QoL.
Primary Palliative Care
We identified one RCT of a primary palliative care intervention for patients with HF (unclear risk of bias due to potential concerns regarding intervention fidelity; Table 4). Within four Veterans Affairs Medical Centers, Bekelman and colleagues compared the effectiveness of a collaborative care management and telemonitoring intervention versus usual care (55). At one-year follow-up, there was no significant difference between groups regarding QoL (primary outcome). Among secondary outcomes, there was no difference in rates of hospital readmission (29.4% vs. 29.9%, p=0.87). Although one-year mortality was decreased among the intervention group (4.3% vs. 9.6%, p=0.04), this finding should be viewed as preliminary given that it was a secondary outcome.
Table 4.
Summary of Existing Trials of Primary Palliative Care/Collaborative Care Models in Heart Failure
| Study (Country) | Patient Population* | Intervention (Participants Randomized) | Control (Participants Randomized) | Results | Risk of Bias | |
|---|---|---|---|---|---|---|
| Subjective | Objective | |||||
| Bekelman et al, 2015 (USA) | HF with poor QOL, limited functional status, and significant symptoms (KCCQ score <60) (Mean age: 68) | Multidisciplinary collaborative HF disease management, and tele- monitoring with patient self-care support (n=187) | Usual care (n=197) |
QOL [KCCQ]: NS at 1 year; 54.2 (95% CI, 51.7 to 56.6) vs. 53.6 (95% CI, 51.1 to 56.0) Mortality: Decreased at 1 year; 4.3% vs. 9.67%, P=0.04 Mood [PHQ- 9]: Improvement in depression among patients with initial positive screen; mean difference, 2.1-point reduction (95% CI, 0.43 to 3.78); P=0.01 Hospital readmission: NS at 1 year; 29.4% vs. 29.9%; P=0.87 |
Unclear | Unclear |
HF= Heart Failure. KCCQ= Kansas City Cardiomyopathy Questionnaire. NS=Not significant. PHQ-9= Patient Health Questionnaire 9. QOL=Quality of Life. USA=United States of America.
Patient population details the indication for palliative care. All comparisons stated as intervention vs. control.
Summary of evidence
Six palliative care intervention trials met inclusion criteria. Although the evidence base for palliative care in HF is nascent, there is somewhat consistent evidence that a palliative approach improves a variety of patient-centered outcomes, including symptom burden and QoL. Nevertheless, it is clear that research regarding palliative care in HF is still developing, and due to concerns regarding risk of bias in the majority of included trials, conclusions should be interpreted cautiously. Yet, recent evidence from the high-quality PAL-HF trial provides support for the notion that longitudinal palliative care, provided concomitantly with usual HF management, is associated with improved patient-centered outcomes (45).
EXISTING CLINICAL GUIDELINES REGARDING PALLIATIVE CARE IN HF
There are growing numbers of guidelines from major cardiology societies, including the American College of Cardiology Foundation (ACCF), American Heart Association (AHA), International Society for Heart and Lung Transplantation (ISHLT), the Heart Rhythm Society, and Heart Failure Society of America (HFSA), encouraging the incorporation of palliative care into the care of patients with HF. Historically, most of these guidelines have focused on end-of-life decision making with respect to device management, including implantable cardiac defibrillators (ICD) and mechanical circulatory support, or referral to hospice. More recently, there has been an acknowledgement of the benefits of palliative care earlier in the disease trajectory(39).
Several guidelines also advocate that the HF and specialty palliative care teams jointly help patients and families decide on treatment options, with an emphasis on decision-making in the context of advanced HF. For example, the 2013 ISHLT statement recommended that specialty palliative care consultation should be included in the treatment of end-stage HF during the evaluation phase for mechanical circulatory support, and that in addition to managing symptoms, clinicians should be having discussions about goals and preferences for end-of-life care with patients receiving mechanical circulatory support as destination therapy (41). In 2012, AHA experts recommended referral to specialty palliative care for assistance with difficult decision making, symptom management in advanced disease, and caregiver support, emphasizing that “the use of palliative care services should not be considered equivalent to the withdrawal of disease-modifying therapies.”(40) A 2015 HFSA statement also recommended incorporating specialty palliative and hospice care into patients with advanced HF care plans, specifying that decision making should include the patient’s wishes for survival improvement versus QoL optimization (42).
The above recommendations have recently expanded into The Joint Commission (TJC) and the Centers for Medicare & Medicaid Services (CMS) mandates. As of October 30, 2014, TJC revised its requirements for disease-specific advanced certification program for Ventricular Assist Device (VAD) for Destination Therapy (DT)(56) and specifically added a requirement to include a specialty palliative care representative to the core interdisciplinary team. Following TJC updates, CMS published its final memorandum for VADs for Bridge-to-Transplant (BTT) and DT, again mandating the inclusion of palliative care specialists in the multidisciplinary team of medical professionals caring for beneficiaries receiving VADs as DT (57).
As illustrated, multiple guidelines advocate for the involvement of specialty palliative care in decisions regarding high-technology interventions and end-of-life care. However, there is little emphasis on (1) addressing the many domains of patient and family QoL aside from functional status, (2) integrating palliative care earlier in the HF trajectory, or (3) providing palliative care concurrently with HF-directed therapies, particularly for patients who are ineligible for or who prefer not to receive cardiac devices.
PRIORITIES FOR FUTURE RESEARCH AND CLINICAL IMPLEMENTATION OF PALLIATIVE CARE IN HF
To date, the rationale for palliative care in HF has largely been one of analogy from the benefits reported from studies of palliative care in oncology. Yet, it is neither likely nor appropriate to assume that the framework of palliative care used in oncology is optimal for patients living with chronic, non-malignant illnesses, such as HF. Indeed, the next era of palliative care research and clinical implementation will challenge the status quo of palliative care, both in terms of content and structure, to maximize impact and uptake in chronic illness.(58) Whereas few randomized trials of palliative care interventions exist in HF, as we have illustrated, these trials are an important yet imperfect starting point for future investigation. Three critical questions remain unanswered in the literature representing the next priorities in explicating the role of palliative care in HF.
First, how do we build capacity in addressing the unmet palliative needs of patients with HF? True innovation regarding the ability to disseminate and sustain palliative care will disrupt the prevailing reliance on the increasingly scarce resource of palliative care specialists(59). Indeed, all clinicians caring for patients with serious illness, like HF, should possess a fundamental palliative proficiency to alleviate suffering (e.g., basic management of physical and psychological symptoms, eliciting goals of care, responding to family concerns) (14). Initial efforts to educate cardiology fellows in palliative care competencies, such as communication, are underway (60,61). Research is needed to understand how to improve education regarding primary palliative care domains that are relevant to patients with advanced HF, such as elicitation of goals of care, advance care planning, and caregiver support. As a result, not only is palliative care normalized, it is also able to be provided seamlessly and longitudinally across the HF experience – not solely in the inpatient setting in response to acute decompensation crises. Indeed, multiple aspects of palliative care (e.g., symptom self-management, care coordination, decision support, patient activation) align with principles of disease management and HF self-care (62,63). For example, primary clinicians should provide proactive education and support to patients to promote self-management of burdensome symptoms, while offering specialty palliative care resources as an option if these needs become intractable. We present suggested roles for primary and specialty palliative care in HF in Table 5. Yet these considerations for primary palliative care in HF are largely theoretical (58); research is needed to examine integrating primary palliative care within primary care and cardiology settings, reserving specialty palliative care for patients with complex needs.
Table 5.
Primary palliative care versus specialist palliative care for patients with HF and their families
| Domain | Primary Palliative Care (PPC) | When to refer to specialist palliative care (SPC) | |
|---|---|---|---|
| Symptom Management | Shortness of Breath |
|
|
| Pain |
|
|
|
| Depressed Mood |
|
|
|
| Anxiety |
|
|
|
| Nausea |
|
|
|
| Fatigue |
|
|
|
| Insomnia |
|
|
|
| Communication and Advance Care Planning | Discussing code status |
|
|
| Advance care planning, including decisions to withdraw life- sustaining therapies |
|
|
|
| LVAD Preparedness Planning/Transplant Decisions |
|
|
|
| Request for assisted suicide |
|
|
|
| Psychological support | Patient support |
|
|
| Caregiver support |
|
|
|
| Care Coordination |
|
|
|
Adapted with permission from: Gelfman, L.P., Kavalieratos, D., Teuteberg, W.G. et al. Heart Fail Rev (2017). doi:10.1007/s10741-017-9604-9
Abbreviations: HF, heart failure. PPC, primary palliative care. SPC, specialty palliative care. LVAD, left ventricular assist device. TJC, The Joint Commission. CPR, cardiopulmonary resuscitation.
Second, which palliative care models and delivery methods are most effective in optimizing outcomes for a particular patient with HF? Trials are needed to identify the comparative effectiveness of various permutations of palliative care delivery in HF, specifically across two characteristics: provider specialization (e.g., primary care vs. cardiology vs. palliative care) and delivery method (e.g., in person vs. telephonic vs. video-based). First, although more studies are needed to confirm the effectiveness of primary palliative care in HF, subsequent trials must directly compare this model with specialty palliative care management. Whereas intuition would argue for the relative superiority of specialty palliative care over a primary palliative approach, this assumption remains untested, and, as noted previously, access to specialty care for all HF patients is considerably limited. Second, while evidence suggests that telephone-based palliative care is effective in oncology (64), no head-to-head trial has evaluated this model against in-person palliative care. It is unclear whether palliative care delivered remotely is equivalent to the arguably more resource-intensive method of in-person consultation. To ensure maximal relevance, these studies must simultaneously assess patient (e.g., QoL, symptom burden), caregiver (e.g., burden, mood), and health system outcomes (e.g., utilization, costs).
Third, which treatments are most effective for addressing symptom burden in patients with HF? Although the most common symptoms for patients with HF are well known to be depression, anxiety, sleep disturbance, fatigue, dyspnea, and pain (48,65,66), additional studies are needed to expand the range of effective treatment modalities for these symptoms. For example, recent intervention studies of psychiatric comorbidity in HF have failed to yield a clear conclusion of the effectiveness of treatments (67). This is likely due to differences in the underlying pathophysiology of these symptoms, which may differ in cardiac versus non-cardiac conditions (68,69). Given this gap in the literature, it remains challenging to effectively treat these very burdensome symptoms. Furthermore, due to often extreme medical complexity and frailty in this population, it can often be additionally difficult to discern a distinct symptom from progression of the overall disease process (e.g. fatigue due to depression or due to HF). Relatedly, the severity of perceived symptoms notoriously reflects poorly the degree of underlying cardiac pathophysiology. For example, dyspnea is experienced in up to 90% of patients with HF(70), yet this is frequently in the absence of hypoxemia or hypercapnia (71). In addition, physiological measures of disease severity, such as ejection fraction, may be inadequate proxies for health status and other subjective markers of well-being (72). Idiosyncrasies such as these further complicate studies of potential palliative treatments due to the difficulty of establishing appropriate subject inclusion and response criteria for these symptoms.
CONCLUSION
Although the evidence base for palliative care in HF is in its infancy(73,74), interest in this area continues to proliferate as evidenced by the recent publication of the groundbreaking PAL-HF trial, as well as multiple clinical trials also underway examining various forms of palliative care delivery in HF. Given the growing prevalence of HF, the integration of palliative care within HF management represents an opportunity to affect the public health issue of poor QoL in patients and caregivers, while also optimizing care delivery. Furthermore, research and clinical implementation of palliative care in HF can serve as a vanguard for explicating the role of palliative care in other chronic, non-malignant illnesses.
Acknowledgments
The authors sincerely thank Judith Resick, MSN MPH RN for her assistance with editing, as well as Winifred Teuteberg, MD for her assistance with the creation of Table 4. We also thank our collaborators who assisted with the systematic review from which this manuscript draws: Jennifer Corbelli, MD MS; Di Zhang, BS; J. Nicholas Dionne-Odom, PhD RN; Natalie C. Ernecoff, MPH; Janel Hanmer, MD PhD; Zachariah P. Hoydich, BS; Michele Klein-Fedyshin, MSLS BSN RN BA; Camilla Zimmermann, MD PhD; Sally C. Morton, PhD; Lucas Heller, MD; and, Yael Schenker, MD MAS.
Funding: Dr. Kavalieratos receives research support from the NHLBI (K01-HL133466). Dr. Gelfman was supported by a K23 (K23-AG049930) from the NIA. Dr. Goldstein is supported by funds from the Mount Sinai Claude D. Pepper Older Americans Independence Center (P30-AG028741) and a grant from the NHLBI (R01-HL102084). Dr. Bekelman receives research support from the Department of Veterans Affairs (VA) and the VA Eastern Colorado Health Care System. Dr. Bakitas is supported by a grant from NINR (R01-NR013665). IMPACT-HF2 (Improve Palliative Care Therapies for Patients with Heart Failure and Their Families), a working group of palliative care and heart failure experts was supported by the American Federation for Aging Research, the John A. Hartford Foundation, the National Palliative Care Research Center, Mount Sinai’s Claude Pepper Older American Independence Center (P30-AG028741), and University of Alabama at Birmingham Centers for Comprehensive Cardiovascular Care and Palliative and Supportive Care.
Abbreviations
- BTT
Bridge-to-transplant
- CI
confidence interval
- CMS
Centers for Medicare & Medicaid Services
- DT
destination therapy
- ED
emergency department
- HF
heart failure
- ICD
Implantable cardiac device
- QoL
quality of life
- RCT
randomized clinical trial
- VAD
ventricular assist device
Footnotes
Disclaimer: The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Writing Group M. Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Eisen HJ. Epidemiology of heart failure and scope of the problem. Cardiol Clin. 2014;32:1–8. vii. doi: 10.1016/j.ccl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Solano J, Gomes B, Higginson I. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13:643–648. doi: 10.1016/j.cardfail.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Goebel J, Doering L, Shugarman L, et al. Heart failure: the hidden problem of pain. Journal of pain and symptom management. 2009;38:698–707. doi: 10.1016/j.jpainsymman.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evangelista L, Sackett E, Dracup K. Pain and heart failure: unrecognized and untreated. Eur J Cardiovasc Nurs. 2009;8:169–173. doi: 10.1016/j.ejcnurse.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cain CJ, Wicks MN. Caregiver attributes as correlates of burden in family caregivers coping with chronic obstructive pulmonary disease. Journal of Family Nursing. 2000;6:46–68. [Google Scholar]
- 8.Dracup K, Evangelista LS, Doering L, Tullman D, Moser DK, Hamilton M. Emotional well-being in spouses of patients with advanced heart failure. Heart & lung : the journal of critical care. 2004;33:354–61. doi: 10.1016/j.hrtlng.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Harding R, Selman L, Beynon T, et al. Meeting the communication and information needs of chronic heart failure patients. J Pain Symptom Manage. 2008;36:149–156. doi: 10.1016/j.jpainsymman.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Lemond L, Allen LA. Palliative care and hospice in advanced heart failure. Prog Cardiovasc Dis. 2011;54:168–78. doi: 10.1016/j.pcad.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison RS, Meier DE. Palliative care. The New England Journal of Medicine. 2004;350:2582–90. doi: 10.1056/NEJMcp035232. [DOI] [PubMed] [Google Scholar]
- 13.Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA : the journal of the American Medical Association. 2016;316:2104–2114. doi: 10.1001/jama.2016.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. The New England journal of medicine. 2013;368:1173–5. doi: 10.1056/NEJMp1215620. [DOI] [PubMed] [Google Scholar]
- 15.Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The Growth of Palliative Care in U.S. Hospitals: A Status Report. Journal of palliative medicine. 2016;19:8–15. doi: 10.1089/jpm.2015.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier DE, Beresford L. Outpatient clinics are a new frontier for palliative care. J Palliat Med. 2008;11:823–8. doi: 10.1089/jpm.2008.9886. [DOI] [PubMed] [Google Scholar]
- 17.Kavalieratos D, Mitchell EM, Carey TS, et al. “Not the ‘grim reaper service’”: An assessment of provider knowledge, attitudes, and perceptions regarding palliative care referral barriers in heart failure. J Amer Heart Assn. 2014;3:e544–e544. doi: 10.1161/JAHA.113.000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–96. doi: 10.1016/j.jacc.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 19.Bekelman DB, Nowels CT, Allen LA, Shakar S, Kutner JS, Matlock DD. Outpatient palliative care for chronic heart failure: a case series. Journal of palliative medicine. 2011;14:815–21. doi: 10.1089/jpm.2010.0508. [DOI] [PubMed] [Google Scholar]
- 20.Alpert CM, Smith MA, Hummel SL, Hummel EK. Symptom burden in heart failure: assessment, impact on outcomes, and management. Heart Fail Rev. 2017;22:25–39. doi: 10.1007/s10741-016-9581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kavalieratos D, Mitchell EM, Carey TS, et al. “Not the ‘grim reaper service’”: an assessment of provider knowledge, attitudes, and perceptions regarding palliative care referral barriers in heart failure. J Am Heart Assoc. 2014;3:e000544. doi: 10.1161/JAHA.113.000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–37. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 23.Saveanu RV, Mayes T. Diagnosing depression in congestive heart failure. Heart Fail Clin. 2011;7:75–9. doi: 10.1016/j.hfc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor CM, Joynt KE. Depression: are we ignoring an important comorbidity in heart failure? J Am Coll Cardiol. 2004;43:1550–2. doi: 10.1016/j.jacc.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Cagle JG, Bunting M, Kelemen A, Lee J, Terry D, Harris R. Psychosocial needs and interventions for heart failure patients and families receiving palliative care support: a systematic review. Heart Fail Rev. 2017 doi: 10.1007/s10741-017-9596-5. [DOI] [PubMed] [Google Scholar]
- 26.Hooker SA, Grigsby ME, Riegel B, Bekelman DB. The Impact of Relationship Quality on Health-Related Outcomes in Heart Failure Patients and Informal Family Caregivers: An Integrative Review. J Cardiovasc Nurs. 2015;30:S52–63. doi: 10.1097/JCN.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 27.Nicholas Dionne-Odom J, Hooker SA, Bekelman D, et al. Family caregiving for persons with heart failure at the intersection of heart failure and palliative care: a state-of-the-science review. Heart Fail Rev. 2017 doi: 10.1007/s10741-017-9597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bekelman DB, Rumsfeld JS, Havranek EP, et al. Symptom burden, depression, and spiritual well-being: a comparison of heart failure and advanced cancer patients. J Gen Intern Med. 2009;24:592–8. doi: 10.1007/s11606-009-0931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setoguchi S, Glynn RJ, Stedman M, Flavell CM, Levin R, Stevenson LW. Hospice, opiates, and acute care service use among the elderly before death from heart failure or cancer. American heart journal. 2010;160:139–44. doi: 10.1016/j.ahj.2010.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakas T, Pressler SJ, Johnson EA, Nauser JA, Shaneyfelt T. Family caregiving in heart failure. Nurs Res. 2006;55:180–8. doi: 10.1097/00006199-200605000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Goodlin SJ, Hauptman PJ, Arnold R, et al. Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail. 2004;10:200–9. doi: 10.1016/j.cardfail.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–96. doi: 10.1016/j.jacc.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 33.Janssen DJ, Spruit MA, Wouters EF, Schols JM. Daily symptom burden in end-stage chronic organ failure: a systematic review. Palliat Med. 2008;22:938–48. doi: 10.1177/0269216308096906. [DOI] [PubMed] [Google Scholar]
- 34.Goodlin SJ. End-of-life care in heart failure. Curr Cardiol Rep. 2009;11:184–91. doi: 10.1007/s11886-009-0027-7. [DOI] [PubMed] [Google Scholar]
- 35.Yim CK, Barron Y, Moore S, et al. Hospice Enrollment in Patients With Advanced Heart Failure Decreases Acute Medical Service Utilization. Circ Heart Fail. 2017:10. doi: 10.1161/CIRCHEARTFAILURE.116.003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costanzo MR, Mills RM, Wynne J. Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM) American heart journal. 2008;155:339–47. doi: 10.1016/j.ahj.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Unroe KT, Greiner MA, Hernandez AF, et al. Resource use in the last 6 months of life among medicare beneficiaries with heart failure, 2000–2007. Arch Intern Med. 2011;171:196–203. doi: 10.1001/archinternmed.2010.371. [DOI] [PubMed] [Google Scholar]
- 38.Cheung WY, Schaefer K, May CW, et al. Enrollment and events of hospice patients with heart failure vs. cancer. J Pain Symptom Manage. 2013;45:552–60. doi: 10.1016/j.jpainsymman.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125:1928–52. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–87. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Fang JC, Ewald GA, Allen LA, et al. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. Journal of cardiac failure. 2015;21:519–34. doi: 10.1016/j.cardfail.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 43.McKelvie RS, Moe GW, Cheung A, et al. The 2011 Canadian Cardiovascular Society heart failure management guidelines update: focus on sleep apnea, renal dysfunction, mechanical circulatory support, and palliative care. Can J Cardiol. 2011;27:319–38. doi: 10.1016/j.cjca.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Dahlin C. National Consensus Project for Quality Palliative Care. Clinical practice guidelines for quality palliative care. 2013 doi: 10.1007/s10730-010-9128-3. [DOI] [PubMed] [Google Scholar]
- 45.Rogers JG, Patel CB, Mentz RJ, et al. Palliative Care in Heart Failure: The PAL-HF Randomized, Controlled Clinical Trial. J Am Coll Cardiol. 2017;70:331–341. doi: 10.1016/j.jacc.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 ed. The Cochrane Collaboration; 2011. [Google Scholar]
- 47.Evangelista LS, Lombardo D, Malik S, Ballard-Hernandez J, Motie M, Liao S. Examining the effects of an outpatient palliative care consultation on symptom burden, depression, and quality of life in patients with symptomatic heart failure. J Card Fail. 2012;18:894–9. doi: 10.1016/j.cardfail.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bekelman DB, Nowels CT, Allen LA, Shakar S, Kutner JS, Matlock DD. Outpatient Palliative Care for Chronic Heart Failure: A Case Series. J Palliat Med. 2011;14:815–821. doi: 10.1089/jpm.2010.0508. [DOI] [PubMed] [Google Scholar]
- 49.Dionne-Odom JN, Kono A, Frost J, et al. Translating and testing the ENABLE: CHF-PC concurrent palliative care model for older adults with heart failure and their family caregivers. J Palliat Med. 2014;17:995–1004. doi: 10.1089/jpm.2013.0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewin WH, Schaefer KG. Integrating palliative care into routine care of patients with heart failure: models for clinical collaboration. Heart failure reviews. 2017 doi: 10.1007/s10741-017-9599-2. [DOI] [PubMed] [Google Scholar]
- 51.Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. Journal of palliative medicine. 2015;18:134–42. doi: 10.1089/jpm.2014.0192. [DOI] [PubMed] [Google Scholar]
- 52.Hopp FP, Zalenski RJ, Waselewsky D, et al. Results of a Hospital-Based Palliative Care Intervention for Patients With an Acute Exacerbation of Chronic Heart Failure. Journal of cardiac failure. 2016;22:1033–1036. doi: 10.1016/j.cardfail.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Wong F, Ng A, Lee P, et al. Effects of a transitional palliative care model on patients with end-stage heart failure: A randomised controlled trial. Heart. 2016 doi: 10.1136/heartjnl-2015-308638. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brannstrom M, Boman K. Effects of person-centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. European journal of heart failure. 2014;16:1142–51. doi: 10.1002/ejhf.151. [DOI] [PubMed] [Google Scholar]
- 55.Bekelman DB, Plomondon ME, Carey EP, et al. Primary Results of the Patient-Centered Disease Management (PCDM) for Heart Failure Study: A Randomized Clinical Trial. JAMA internal medicine. 2015;175:725–32. doi: 10.1001/jamainternmed.2015.0315. [DOI] [PubMed] [Google Scholar]
- 56.Modified: Ventricular assist device destination therapy requirements. Jt Comm Perspect. 2014;34:6–7. [PubMed] [Google Scholar]
- 57.Proposed Decision Memo for Ventricular Assist Devices for Bridge-to-Transplant and Destination Therapy (CAG-00432R). 2013.
- 58.Kavalieratos D, Rollman BL, Arnold RM. Homeward Bound, not hospital rebound: how transitional palliative care can reduce readmission. Heart. 2016;102:1079–80. doi: 10.1136/heartjnl-2016-309385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lupu D American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40:899–911. doi: 10.1016/j.jpainsymman.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Munoz-Mendoza J. Competencies in palliative care for cardiology fellows. J Am Coll Cardiol. 2015;65:750–2. doi: 10.1016/j.jacc.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 61.Berlacher K, Arnold RM, Reitschuler-Cross E, Teuteberg J, Teuteberg W. The Impact of Communication Skills Training on Cardiology Fellows’ and Attending Physicians’ Perceived Comfort with Difficult Conversations. J Palliat Med. 2017;20:767–769. doi: 10.1089/jpm.2016.0509. [DOI] [PubMed] [Google Scholar]
- 62.Krumholz HM, Currie PM, Riegel B, et al. A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation. 2006;114:1432–45. doi: 10.1161/CIRCULATIONAHA.106.177322. [DOI] [PubMed] [Google Scholar]
- 63.Riegel B, Dickson VV, Faulkner KM. The Situation-Specific Theory of Heart Failure Self-Care: Revised and Updated. J Cardiovasc Nurs. 2016;31:226–35. doi: 10.1097/JCN.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 64.Bakitas, Lyons M, Hegel KD, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302:741–9. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kavalieratos D, Kamal AH, Abernethy AP, et al. Comparing unmet needs between community-based palliative care patients with heart failure and patients with cancer. J Palliat Med. 2014;17:475–81. doi: 10.1089/jpm.2013.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nordgren L, Sorensen S. Symptoms experienced in the last six months of life in patients with end-stage heart failure. Eur J Cardiovasc Nurs. 2003;2:213–217. doi: 10.1016/S1474-5151(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 67.Ströhle A, Rieckmann N. Escitalopram and Outcomes Among Patients With Depression and Heart Failure. JAMA. 2016;316:1494–1494. doi: 10.1001/jama.2016.13855. [DOI] [PubMed] [Google Scholar]
- 68.Beattie J, Goodlin S. Supportive care in heart failure. Oxford University Press; 2011. [Google Scholar]
- 69.Xiong GL, Fiuzat M, Kuchibhatla M, et al. Health status and depression remission in patients with chronic heart failure: patient-reported outcomes from the SADHART-CHF trial. Circ Heart Fail. 2012;5:688–92. doi: 10.1161/CIRCHEARTFAILURE.112.967620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nordgren L, Sorensen S. Symptoms experienced in the last six months of life in patients with end-stage heart failure. Eur J Cardiovasc Nurs. 2003;2:213–7. doi: 10.1016/S1474-5151(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 71.Goodlin SJ. Palliative care in congestive heart failure. Journal of the American College of Cardiology. 2009;54:386–396. doi: 10.1016/j.jacc.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 72.Heidenreich PA, Spertus JA, Jones PG, et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47:752–6. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 73.Xie K, Gelfman L, Horton JR, Goldstein NE. State of Research on Palliative Care in Heart Failure as Evidenced by Published Literature, Conference Proceedings, and NIH Funding. Journal of cardiac failure. 2017;23:197–200. doi: 10.1016/j.cardfail.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McIlvennan CK, Allen LA. Palliative care in patients with heart failure. BMJ. 2016;353:i1010. doi: 10.1136/bmj.i1010. [DOI] [PubMed] [Google Scholar]