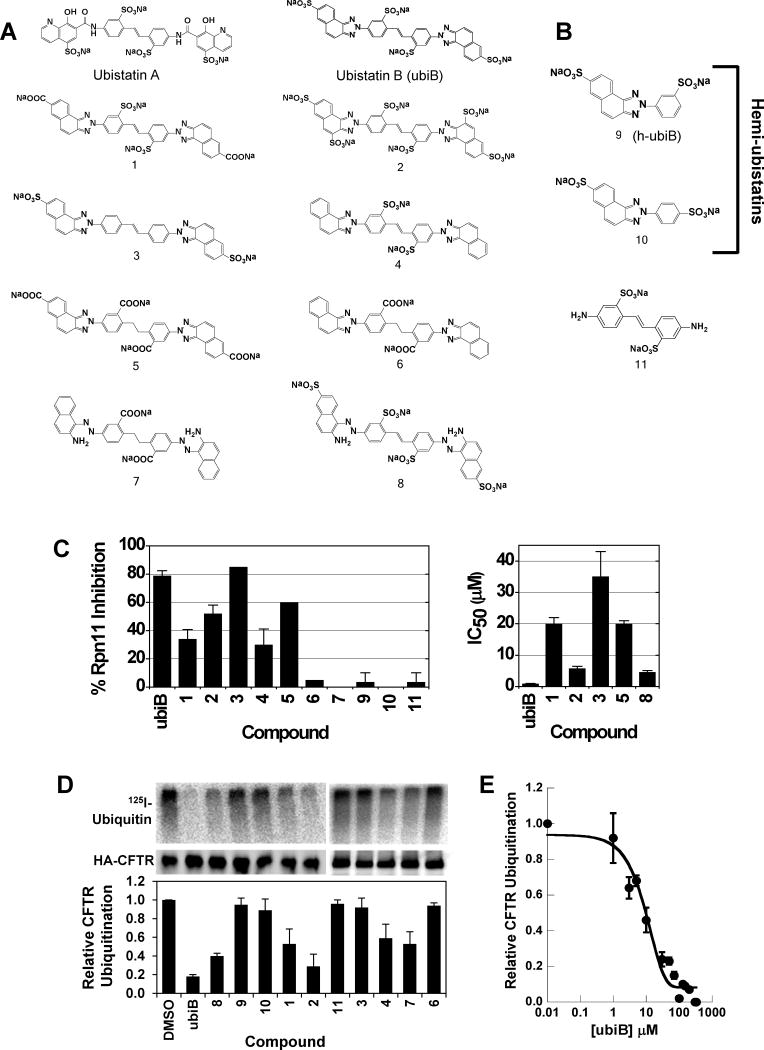
Figure 1.
Chemical structures of various synthesized ubistatin derivatives and the development of a structure-activity relationship. (A) Full-ubistatin compounds and (B) hemi-ubistatins. (C) The effect of ubistatin variants on the DUB activity of Rpn11 in purified proteasome: percent inhibition measured at 10 μM (left) and IC50 (right). Data represent the mean and standard deviation (error bars) determined from multiple measurements. See also Table S1. (D) In vitro CFTR ubiquitination reactions in the presence of the indicated ubistatin compounds. Ubiquitinated CFTR (top) is quantified relative to the amount of HA-tagged CFTR protein present in the immunoprecipitate (middle). The results are tabulated in the graph (bottom) and Table S2. (E) Dose-dependent effect of ubistatin B (ubiB) on CFTR ubiquitination. In vitro CFTR ubiquitination reactions were performed as in (D) with the final concentrations of ubiB as shown. The reactions with 0.01 μM ubiB were set to 100% and the extent of CFTR ubiquitination was equivalent to that of a DMSO control (not shown). Data represent the mean and standard deviation (error bars) of at least 3 determinations. See also Table S1.