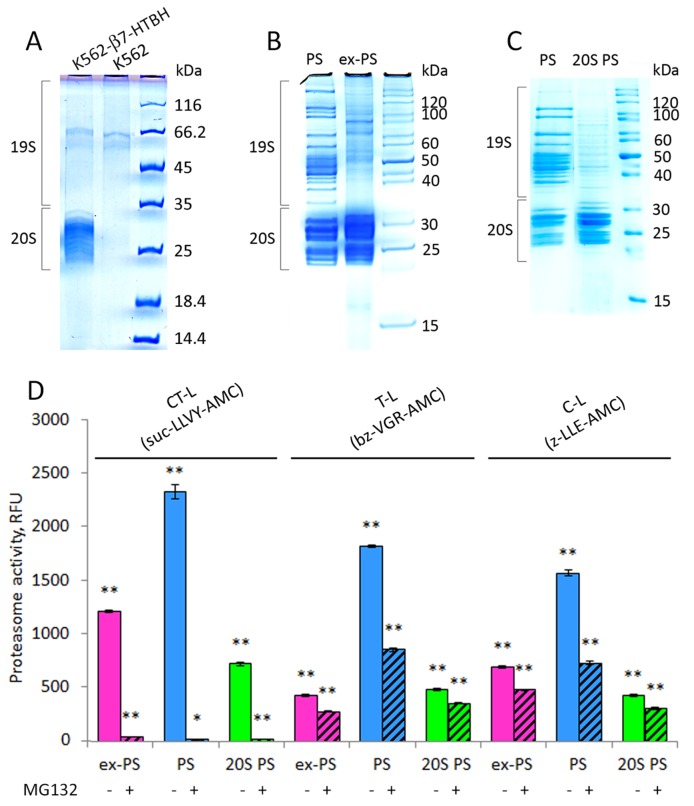
Figure 5. Affinity-purified proteasomes from conditioned medium (CM) and β7-HTBH K562 cells preserve chymotrypsin-like (CT-L) peptidase activity but decrease trypsin- and caspase-like (T-L and C-L) activities.
(A) 1D SDS-PAGE patterns of the purified samples from CM of control K562 cells and stable cell line expressing the β7-HTBH. (B) Proteins from affinity-purified cellular (PS) and extracellular (ex-PS) proteasomes (15 μg) were separated by SDS-PAGE, visualized with Coomassie Blue. Positions of 19S and 20S subcomplexes in the gel are shown. (C) Affinity-purified PS were dissociated into 20S (20S PS) and 19S subcomplexes by 1M NaCl treatment and then separated by SDS-PAGE followed by staining with Coomassie Blue. (D) Chymotrypsin- (CT-), trypsin- (T-), and caspase- (C-) like activities of ex-PS (1 μg) in comparison with PS in the presence or absence of proteasome inhibitor MG132 were determined by fluorometric quantification of the substrates Suc-LLVY-AMC, Ac-RLR-AMC and Z-LLE-AMC, using 380 nm excitation/440 nm emission, respectively. The results are given as Relative fluorescence units. Values shown are mean ± standard deviation from three independent experiments (*p<0.01, **p<0.001).