Abstract
This systematic review and meta-analyses investigates the expression of the cell checkpoint regulator, mitotic arrest deficiency protein 2 (MAD2) in cancerous tissue and examines whether an association exists between MAD2 levels and cancer survival and recurrence. Studies investigating MAD2 expression in cancer tissue utilising immunohistochemistry (IHC) were identified by systematic literature searches of Medline, Embase and Web of Science databases by October 2015. Random effects meta-analyses were performed to generate pooled hazard ratios (HRs) with 95% confidence intervals (CIs) of overall and progression-free survival according to MAD2 expression. Forty-three studies were included in the overall review. In 33 studies investigating MAD2 expression by IHC in cancer tissue, a wide range of expression positivity (11–100%) was reported. Higher MAD2 expression was not associated with an increased risk of all-cause mortality in a range of cancers (pooled HR 1.35, 95% CI 0.97–1.87; P = 0.077, n = 15). However, when ovarian cancer studies were removed, a significant pooled HR of 1.59 for risk of all-cause mortality in other cancer patients with higher expressing MAD2 tumours was evident (95% CI, 1.17–2.17; P = 0.003, n = 12). In contrast, higher MAD2 expression was associated with significant decreased risk of all-cause mortality in ovarian cancer patients (pooled HR = 0.50, 95% CI, 0.25–0.97; P = 0.04, n = 3). In conclusion, with the exception of ovarian cancer, increased MAD2 expression is associated with increased risk of all-cause mortality and recurrence in cancer. For ovarian cancer, reduced levels of MAD2 are associated with poorer outcome. Further studies are critical to assess the clinical utility of a MAD2 IHC biomarker.
Keywords: MAD2, cancer, prognosis and systematic review
INTRODUCTION
The mitotic arrest deficiency protein 2 (MAD2) is an essential spindle assembly checkpoint (SAC) monitoring accurate chromosomal alignment at the metaphase plate before mitosis [1]. Much evidence implicates the overexpression of MAD2 as a driver of chromosomal instability in many cancer types [2–5]. Tumour formation resulting from loss of one of the MAD2 alleles suggests haploinsufficiency at this locus which is common to other SAC proteins [6]. MAD2 also functions beyond the SAC where it is known to interact with a number of checkpoint-unrelated proteins such as insulin receptor, estrogen receptor β and tumour necrosis factor α convertase [7–9]. Moreover, MAD2 influences cell proliferation, tumour metastasis and tumour recurrence [5].
MAD2 expression is regulated by a diverse range of cancer-associated proteins including the tumour suppressor protein p53, breast cancer 1 (BRCA1), the c-MYC oncogene, the checkpoint with forkhead and ring finger domains, E3 ubiquitin protein ligase (CHFR), E2 factor (E2F) and numerous microRNAs [10–16]. Moreover, MAD2 is a ubiquitously expressed protein, expressed by most tissues of the body, found localised in the nuclear, perinuclear and cytoplasmic compartments [17–19]. It is reliably measured by real-time PCR, western blots or immunohistochemistry (IHC) methods [19, 20]. The majority of studies predominantly evaluate MAD2 expression by IHC.
The association between MAD2 IHC expression and survival has been examined in a diverse range of carcinomas including colorectal, bladder, testicular, breast and ovarian, where both overexpression and low levels of MAD2 expression have been associated with survival, depending on the tumour type [20, 21]. However, inconsistencies in terms of study design, staining site analysed, expression cut-offs and study quality have precluded the interpretations of these study results, and the evaluation of MAD2 as a potential prognostic biomarker of patient survival. Therefore, there is an unmet need to systematically review published data relating to the extent of MAD2 expression in various tissues and the relationship between MAD2 expression evaluated through IHC and cancer prognosis.
The primary aim of this novel review and meta-analyses was to systematically identify research papers that analysed the association between MAD2 IHC staining and cancer patient survival and/or recurrence, and from these studies elucidate whether there is an association between MAD2 expression and cancer patient prognosis by conducting a meta-analysis.
RESULTS
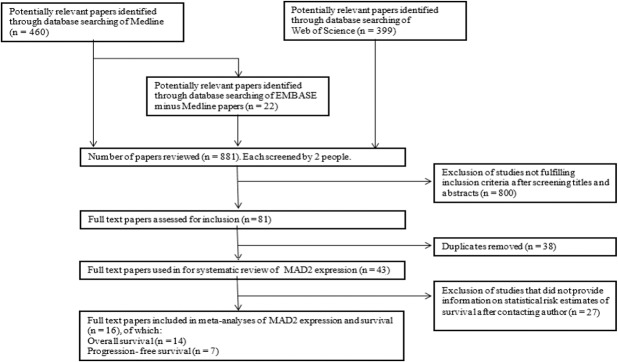
The literature search identified 881 citations for review (Medline n = 460, Embase minus Medline n = 22 and Web of Science n = 399). As shown in Figure 1, after exclusion of publications following screening for title and abstract by reviewers, 81 papers remained and after removal of duplicates, 43 studies were fully screened and included in the review.
Figure 1. Flowchart of study selection that assessed MAD2 immunohistochemistry in cancer.
Three databases (Medline, Embase and Web of Science) were utilised to identify potential papers for inclusion in meta-analysis. After removal of duplicates and studies not fulfilling inclusion criteria, data was extracted from these full text papers to be included in meta-analyses evaluating MAD2 percentage expression and both progression-free and overall survival in relation to MAD2 expression.
MAD2 expression in cancerous tissue
A total of 33 studies provided expression analysis of MAD2 where expression of positivity ranged from 11.1 to 100% in cancerous tissue. Difficulties arose in the comparison of studies due to the fact that the majority of cancer types only have one study. In cancer types where more than one study has been undertaken, comparisons between studies were extremely complicated. This was as a result of the different expression methods utilised and the expression reported as different measures of central tendency (Supplementary Table 1).
Association with overall survival
As shown in Table 1, studies included in this meta-analysis were published between 2008 and 2015, with nine studies originating from East Asia, three studies from Europe, one study from Brazil and one study from the USA. Cancer sample cohorts ranged from 27 to 400. The mean follow-up times, ranged from 3 to 3.29 years with median follow-up times ranging from 1.6 to 5.04 year. MAD2 IHC was mostly evaluated on tissue microarrays (TMAs), one study used both TMAs and full face sections, while six studies did not report the sample type assessed. Monoclonal antibodies were reported in eight studies, three studies reported the use of polyclonal antibodies and three studies did not report the type of antibody used. The cellular location investigated for MAD2 staining was nuclear in six studies, cytoplasmic for two studies, both nuclear and cytoplasmic in five studies and unreported in one study (Table 1). In the IHC staining analysis of MAD2 expression, four studies reported the involvement of a pathologist in the scoring of the IHC slides while 10 studies did not state if a pathologist assisted in the scoring. Two assessors determined MAD2 IHC staining in six studies, a single pathologist assessed MAD2 IHC scoring in one study and the remaining seven studies did not state the number of assessors.
Table 1. Characteristics of studies assessing the expression of MAD2 immunohistochemistry in cancer.
| Author | Year | Cancer site(s) | Samples assessed | Age range, years (mean or median) | Sex | Number of normal tissue samples | Number of cancer samples | Site of staining | Expression analysis | Survival analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Agosten | 2007 | Breast | TMA | NR | Females | NR | 85 | Perinuclear/cytoplasmic | ✓ | X |
| Burum-Auensen | 2008 | Colorectal | TMA | 35–88 (68) | Males & females | 17 | 55 | Nuclear | ✓ | ✓ |
| Burum-Auensen | 2007 | Colorectal (UC-related) |
TMA | NR | NR | 10 | 8 | Nuclear/additional staining was cytoplasmic | ✓ | X |
| Burum-Auensen | 2010 | Testicular | TMA | NR | Males | 21 | 336 | Nuclear with weak staining of cytoplasm | ✓ | X |
| Choi | 2013 | Bladder | TMA | 23–97 (68) | Males & females | NR | 339 | Nuclear & cytoplasmic | ✓ | ✓ |
| Du | 2011 | Breast | NR | 33–83 (54) | Females | NR | 117 | Nuclear/sometimes cytoplasmic | ✓ | X |
| Fung | 2007 | Testicular | NR | 24–67 (37.7) | Males | 12 | 23 | Nuclear & cytoplasmic | ✓ | X |
| Furlong | 2012 | Ovarian | TMA and full face | NR | Females | NR | 82 | Nuclear | ✓ | ✓ |
| Genga | 2015 | Myelodysplastic syndrome | NR | 18–91 (59.5) | Males & females | 10 | 40 | Cytoplasmic | ✓ | ✓ |
| Gladhaug | 2010 | Pancreatic | TMA | NR | Males & females | NR | 218 | Nuclear | ✓ | ✓ |
| Hannisdal | 2010 | Tonsillar | TMA | 43 aged < 60 57 aged ≥ 60 |
Males & females | NR | 105 | Nuclear & cytoplasmic | ✓ | ✓ |
| Hisaoka | 2008 | Sarcoma | NR | NR | NR | 50 | 50 | Nuclear | ✓ | X |
| Kato | 2012 | Lung | TMA | 123 aged < 60 239 aged ≥ 60 |
Males & females | NR | 362 | NR | ✓ | X |
| Kato | 2011 | Lung | TMA | 26–87 (63.5) | Males & females | NR | 358 | Nucleoplasmic | ✓ | ✓ |
| Kim | 2014 | Uterine cervical | TMA | 22–82 (44.1) | Females | 100 | 232 | Nuclear & cytoplasmic | ✓ | ✓ |
| Ko | 2010 | Salivary duct | NR | 42–82 (58.5) | Males & females | NR | 27 | Cytoplasmic | ✓ | ✓ |
| Li | 2013 | Endometrial | NR | 35–85 (51) | Females | 30 normal, 30 hyperplasia | 63 | Nuclear & cytoplasmic | ✓ | ✓ |
| Li | 2003 | Colorectal | NR | 28–81 (54.8) | Males & females | 38 | 38 | Brown-yellow staining mainly in cell plasma | ✓ | X |
| Li | 2004 | Colorectal | NR | 25–79 (52.5) | Males & females | 40 | 40 | Brown-yellow staining mainly in cell plasma | ✓ | ✓ |
| Liao | 2013 | Cervical | NR | 23–65 (43.5) | Females | 10 | 90 | Nuclear & cytoplasmic | ✓ | X |
| McGrogan | 2014 | Ovarian | TMA | 32–77 (52) | Females | NA | 72 | Nuclear | ✓ | ✓ |
| Morishta | 2012 | Uterine cervical | NR | 22–69 | Females | NA | 53 | Nuclear | ✓ | X |
| Nakano | 2012 | Ovarian serous | NR | 26–82 | Females | NA | 41 | Nuclear | ✓ | ✓ |
| Nakano | 2012 | Ovarian mucinous | NR | 13–88 | Females | 30 | 98 | Nuclear | ✓ | ✓ |
| Park | 2013 | Ovarian | NR | 59 aged < 60 26 aged ≥ 60 |
Females | NA | 85 | Nuclear 7 cytoplasmic | ✓ | ✓ |
| Rizzardi | 2014 | Oral SCC | NR | 44–86 (61) | Males & females | NA | 49 | Nuclear | ✓ | ✓ |
| Sotillo | 2006 | Various | TMA | NR | NR | NA | 351 | Nuclear | ✓ | X |
| Suraokar | 2014 | Mesothelioma | TMA | NR | NR | NA | 80 | Nuclear & cytoplasmic | ✓ | ✓ |
| Teixeira | 2015 | Oral cancer | NR | 25 aged < 62, 29 aged ≥ 62 |
Males & females | NA | 54 | Nuclear & cytoplasmic | ✓ | ✓ |
| Thoma | 2009 | Renal | TMA | NR | NR | NA | > 200 | Nuclear | ✓ | X |
| Uemura | 2009 | Esophageal | TMA | 32 aged < 60, 28 aged ≥ 60 |
Males & females | 60 | 60 | Nuclear & cytoplasmic | ✓ | X |
| Wang | 2009 | Gastric | TMA | NR | Males & females | 102 | 102 | Nuclear & cytoplasmic | ✓ | ✓ |
| Yu | 2010 | Osteosarcoma | NR | 12–54 (25.2) | Males & females | 20 | 48 | Nuclear & cytoplasmic | ✓ | ✓ |
| Zhang | 2008 | Hepatocellular | TMA | 184 <median age, 216> median age | Males & females | NR | 400 | Nuclear | X | ✓ |
| Zhao | 2014 | Endometrial | NR | NR (50.8) | Females | 30 | 63 | Nuclear & cytoplasmic | ✓ | X |
NA = not applicable, NR = not reported, TMA = tissue microarray, UC = Ulcerative Colitis.
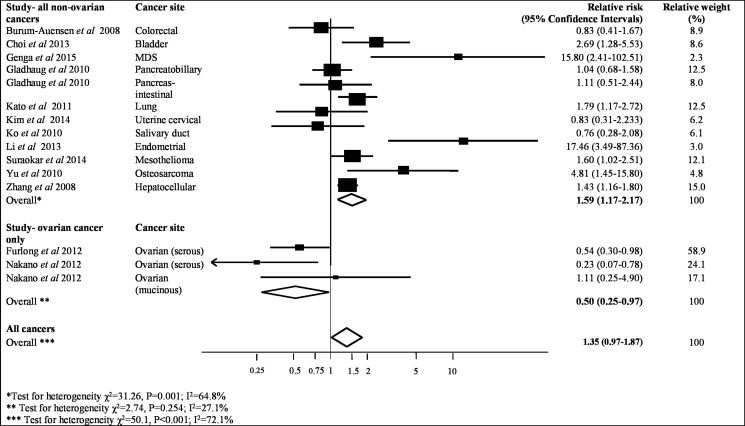
Sufficient data was provided by 14 studies, which contained 2318 patient samples to be included in the meta-analysis, to assess the association between MAD2 expression and overall survival (Table 2). As shown in Figure 2, higher MAD2 expression was associated with a non-significant increased risk of all-cause mortality in a range of cancers (pooled HR 1.35, 95% CI 0.97–1.87; P = 0.08). This investigation had an I2 value of 72% (P < 0.001), highlighting the substantial heterogeneity between studies, which was not markedly reduced in sensitivity analyses excluding individual studies (Figure 2).
Table 2. Descriptive characteristics and results of studies investigating MAD2 expression and cancer progression.
| Author | Cancer site(s) | Comparison (definition)* | Total number of patients/deaths | Mean (max.) follow-up, yrs | Relative risk (95% CI) Overall/cancer-specific survival |
Relative risk (95% CI) Recurrence/disease free survival |
Adjustments |
|---|---|---|---|---|---|---|---|
| Burum-Auensen | Colorectal | High v. low (≥ v. < median %) | 55/NR | NR | HR 0.83, 95%CI 0.41–1.66, P = 0.59 | Multivariate- variables NR | |
| Choi | Bladder | High v. low (>3% v. ≤ 3%, determined by receiver operating characteristic curves) | 339/29 | Mean NR, median was given = 3.08 (15.25) | HR 2.68, 95% CI 1.29–5.55, P = 0.008 | Unadjusted | |
| Furlong | Ovarian | High v. low (intensity 3–4 v. 1–2) | 82/NR | NR | HR 0.54, 95% CI 0.30–0.98, P = 0.041 | HR 0.43, 95% CI 0.24–0.76, P = 0.004 | Stage and grade |
| Genga | Myelodysplastic syndrome | High v. low (≥ 50% v. zero/< 50%) | 40/10 | 3.24 ± 1.7 (5.75) | HR 15.79, 95% CI 2.42–102.86, P = 0.004 | Age, gender, International Prognostic Scoring System at diagnosis | |
| Gladhaug | Pancreatobiliary | High v. low (positive v. negative) | 145/124 | Mean NR, median was given = 1.6 (5) | Unadjusted | ||
| Pancreas - Intestinal | High v. low (positive v. negative) | 73/35 | Mean NR, median was given = 1.6 (5) | HR 1.11, 95% CI 0.51–2.43, P = 0.786 | Unadjusted | ||
| Kato | Lung | High v low (≥ 4 v. ≤ 3) | 358/NR | Mean NR, median given = 5.04 (13.82) | HR 1.78, 95% CI 1.17–2.71, P = 0.008 | HR 1.78, 95% CI 1.17–2.71, P = 0.008 | Age, sex, pT status, pN status, pleural invasion, histological type, smoking history |
| Kim | Uterine cervical | High v. low (≥ 25% v. < 25%) | 232/NR | Mean NR, median was given = 2.75 (10.25) | HR 0.83, 95% CI 0.31–2.23, P = 0.711 | Unadjusted | |
| Ko | Salivary duct | High v. low (intensity 3–4 v. 1–2) | 27/14 | 3.11 (9.75) | HR 0.76, 95% CI 0.28–2.08 P = 0.59 | HR 0.84, 95% CI 0.28–2.51, P = 0.75 | Unadjusted |
| Li | Endometrial | High v. low (positive v. negative) | 63/7 | 3 (5.75) | OR 17.4, 95% CI 3.5–87.62, P = 0.041 | Unadjusted | |
| McGrogan | Ovarian | High vs. low (intensity 3–4 v. 1–2) | 71/44 | NR | HR 0.51, 95% CI 0.23–1.10, P = 0.09 | Tumour stage, grade, and optimal debulking | |
| Nakano | Ovarian serous | High v. low (mean weighted scores 8–12 v. 0–6) | 41/20 | NR | HR 0.23, 95% CI 0.07–0.78, P = 0.019 | HR 0.40, 95% CI 0.15–1.08, P = 0.069 | Unadjusted |
| Nakano | Ovarian mucinous | High v. low (mean weighted scores 8–12 v. 2–6) | 128/NR | NR | HR 1.10, 95% CI 0.25–4.90, P = 0.903 | Unadjusted | |
| Park | Ovarian | High v. low (score ≥ 2 v. ≤ 1) | 85/NR | 2 (6.58) | HR 0.26, 95% CI 0.06–1.09, P = 0.07 | Age, stage, ovarian cancer type, p53 expression | |
| Suraokar | Mesothelioma | High v. low (median, ≥ v. < 44) | 77/75 | NR | HR 1.60, 95% CI 1.02–2.51, P = 0.09 | Unadjusted | |
| Yu | Osteosarcoma | High v. low (strong v. weak) | 48/NR | NR (9) | HR 4.79, 95% CI 1.45–15.80, P < 0.01 | Unadjusted | |
| Zhang | Hepatocellular | High v. low ( ≥10 v. < 10%) | 400/302 | 3.29 (7) | HR 1.44, 95% CI 1.16-1.80, P < 0.001 | Unadjusted |
CI = Confidence intervals, HR = Hazard ratio, NR = not reported, OR: Odds ratio.
Figure 2. Meta-analysis of highest v. lowest category of MAD2 protein levels and risk to overall cancer survival.
Interestingly, when all ovarian cancer studies are removed, as shown in Figure 2, a significant pooled HR of 1.59 for risk of all-cause mortality in patients with high expressing MAD2 tumours was evident compared to those with low expressing MAD2 tumours (95% CI, 1.17–2.17; P = 0.003), but still incurred high heterogeneity (I2 = 65%, P = 0.001). When only ovarian studies were investigated as a separate entity, higher MAD2 expression was associated with a significantly decreased risk of all-cause mortality compared to those with low expressing MAD2 tumours (pooled HR 0.50, 95% CI, 0.25–0.97; P = 0.04). This meta-analysis had an I2 value of 27% (P = 0.25) indicating that the results of the studies are reliably consistent with mild heterogeneity (Figure 2). There was no evidence of publication bias in the funnel plot assessing overall survival for studies (P = 0.82, Supplementary Figure 1). However from undertaking the meta-analysis it is evident that the different ovarian cancer subtypes may respond differently to MAD2 expression levels. Low MAD2 levels correlated with poorer survival as evident in Figure 2. In mucinous ovarian cancers, a different outcome was obtained where low MAD2 levels correlate with decreased risk of all-cause death. However, the association between MAD2 levels and survival in mucinous cancers may not be valid as the HR is extremely close to 1 (HR = 1.11, 95% CI; 0.25–4.90) arising from a single study.
Association with progression-free survival
The meta-analyses of progression-free survival included studies published between 2010 and 2014 with five studies from East Asia and two studies from Europe (Table 1). Cancer sample cohorts ranged from 27 to 358. The mean/median follow-up times reported ranged from 2–5 years.
The use of TMAs or full face sections for MAD2 IHC analysis was unreported in three studies. However, most other studies utilised TMAs with one study using both TMAs and full face sections. Monoclonal antibodies were reported in five studies with two studies not reporting the type used. MAD2 nuclear staining was reported in three studies, MAD2 cytoplasmic staining was reported in one study and both MAD2 nuclear and cytoplasmic staining assessed in two studies (Table 1). MAD2 IHC staining analysis was assessed by a pathologist in two studies, while five studies did not state if a pathologist assisted in the scoring. MAD2 IHC staining was determined by at least two assessors in four studies and three studies did not state the number of assessors.
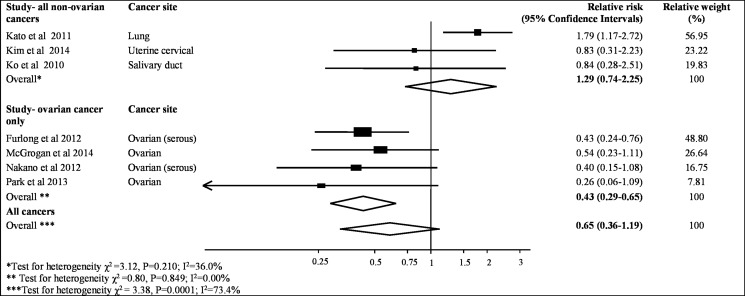
The meta-analysis of the association between MAD2 expression and progression-free survival was assessed from seven studies containing 997 patient samples (Table 2). The results from all studies indicated that lower MAD2 expression was not significantly associated with an increased risk of cancer recurrence (pooled HR = 0.65, 95% CI, 0.36–1.19; P = 0.16, Figure 3). Similar to the association with overall survival, MAD2 expression is different in ovarian cancer compared to other cancers. A pooled HR for progression-free survival in patients with high-expressing non-ovarian MAD2 tumours compared to those with low expressing MAD2 tumours was 1.29 (95% CI, 0.74–2.25; P = 0.38). Mild heterogeneity was observed (I2 value = 36%, P = 0.21). In contrast, a significant pooled HR of 0.43 (95% CI,0.29–0.65; P < 0.001) was evident for progression-free survival in patients with high expressing MAD2 ovarian tumours compared to those with low expressing MAD2 tumours. An I2 value of 0% was seen thereby identifying the results as consistent (P = 0.85). Moreover, the funnel plots for these analyses had minimal asymmetry, indicating no publication bias (P = 0.09, Supplementary Figure 1).
Figure 3. Meta-analysis of highest v. lowest category of MAD2 protein levels and risk to progression free cancer survival.
DISCUSSION
The aim of this novel systematic review with meta-analyses was to summarise the extent of MAD2 expression in various tissues, and to investigate the association between MAD2 IHC expression and both overall and progression-free survival in a diverse range of cancers. The results highlight that higher MAD2 expression is associated with poorer survival in cancer patients, with the exception of ovarian cancer, where conversely, higher MAD2 expression is associated with improved survival.
Our results indicated that MAD2 dysregulation is heavily tissue dependent in cancerous tissue with MAD2 IHC levels ranging from 11.1% to 100% in cancerous tissue. Dysregulation of MAD2 levels by either up- or down-regulation of the gene can result in similar genomic aberrations and contribute to poorer patient survival [14, 40]. From our results, it is evident that high MAD2 levels are associated with increased risk of all-cause death and cancer recurrence in non-ovarian cancers while low MAD2 levels are associate with poorer patient survival in ovarian cancer. Through in vitro and in vivo studies, high MAD2 levels are known to correlate with increased cellular proliferation, cellular transformation, migration, invasiveness and cancer metastasis which could contribute to this poorer patient outcome [41–44]. In addition, a number of studies have shown that low MAD2 levels correlate with resistance to commonly utilised anticancer agents such as taxol, DNA-damaging agents, vincristine and γ- irradiation. Therefore, it is not surprising that both low or high MAD2 levels can lead to a worsen patient prognosis [20, 45–48]. We previously reported that low MAD2 levels correlated with shorter progression-free survival in high grade serous ovarian cancer patients treated with platinum alone or in combination with paclitaxel and concluded this occurred as a result of increased resistance to paclitaxel [20].
In ovarian cancer, the association of high MAD2 expression and survival is in contrast to the reported association of low MAD2 expression in the other cancer types included in this study and raises many questions about why MAD2 levels in ovarian cancer produce such a different response to patient survival. Currently, there is no explanation for this outcome. However, one possible explanation could be linked to the fact that unlike most cancer types that metastasise mainly via haematogenous or lymphatic routes, ovarian cancer, especially the most common and lethal type high grade serous carcinoma, metastasises through peritoneal dissemination [49]. Therefore as ovarian cancers progress and metastasise, they may possibly become less reliant on MAD2 expression as previously reported in osteosarcoma [33]. Another possibility for the dysregulation of MAD2 expression could be related to the regulation of MAD2 by BRCA1 [11]. BRCA1, which is mutated in 10% of ovarian cancers, is a transcriptional regulator of MAD2 and the aberrant activity of mutant BRCA1 in ovarian cancer may lead to altered transcriptional regulation of MAD2 expression [11, 50]. Furthermore, ovarian tumours with high MAD2 levels were previously shown to be associated with increased sensitivity to taxol and improved patient survival, which may indicate that taxol response in ovarian tumours is possibly more dependent on the SAC activity of MAD2 than other cancer types [20, 51].
From the meta-analysis, the different subtypes of ovarian cancer have distinctive survival responses in terms of MAD2 expression. Studies of high grade serous ovarian cancer have shown that low MAD2 levels lead to poorer patient survival. In contrast, mucinous ovarian cancer appears to have a weaker inverse association between MAD2 levels and survival. A possible reasoning for this is that p53 and BRCA1, known regulators of MAD2, are commonly mutated in high serous ovarian cancer (Tp53 mutation is ubiquitous in ovarian high grade serous carcinoma) and mutations in these genes are uncommon in other ovarian cancer subtypes such as mucinous, clear cell, endometrioid and low grade serous [11, 52]. Similarly in breast cancer, where p53 and BRCA1 are also regularly mutated, high MAD2 levels are associated with improved breast cancer specific survival [53]. Additionally, high grade serous ovarian cancers express lower levels of E-cadherin and higher levels of N-cadherin compared to mucinous which is suggestive of a more epithelial-mesenchymal transition phenotype which in-turn triggers tumour metastasis and possibly reduce the requirement of MAD2 for tumour spread [54, 55]. However, due to the limited number of studies on MAD2 expression in ovarian cancer, we cannot conclude if different ovarian subtypes have different survival rates depending on MAD2 expression. Further analysis of MAD2 IHC expression in ovarian cancer may result in an IHC biomarker capable of stratifying the various ovarian cancers subtypes.
The heterogeneity in the meta-analysis of the correlation of MAD2 expression and overall survival in non-ovarian cancer studies was considerable between studies (64.8%). This could be attributable to the methodological diversity between studies, including sub-cellular location evaluated, scoring method utilised, expression threshold chosen and cancer site investigated. However, heterogeneity associated with meta-analyses of MAD2 expression and ovarian cancer prognosis was low.
The primary strength of this review lies in its ability to determine the effect of MAD2 expression on cancer survival for the first time, taken from a robust search of three databases for relevant studies. We were also able to standardise comparator groups, displaying all results as high v. low MAD2 levels, which overcomes potential confusion when comparing results from studies reporting high v. low or low v. high comparisons of MAD2 expression in relation to progression. The utility of prognostic markers are relevant to both understanding the mechanisms of progression and the ability to predict patient outcome (who may benefit from more/less intensive treatments or follow-up regimens). MAD2 could also be combined with other cell cycle proteins such as P27, CDC20, BUBR1. However, there is limited evidence to suggest it would improve the prognostic ability of MAD2 [21, 32, 41, 56, 57]. Furthermore, there was no evidence of publication bias associated with the meta-analyses reported in this study which increases the robustness of the results.
One of the limitations of this review is that currently there is no standardised method for MAD2 IHC evaluation. Standardisation is vital across the entire IHC process from pre- to post-analytical stages and across laboratories to allow comparison between studies [58, 59]. This was particularly noticeable in the evaluation scoring system, where in some cases only MAD2 expression positivity was determined with the degree or percentage of MAD2 staining intensity not considered. Furthermore, the arbitrary cut-off chosen to indicate high MAD2 expression differs from study to study. Between-study variances may also have resulted from diverse populations utilised in these studies especially since a number of the studies were quite small. Standardisation of these factors across studies will allow more comparable studies and provide more robust results. Another limitation is that a meta-analysis for cancer-specific survival according to MAD2 expression was unable to be undertaken due to a lack of studies reporting on this outcome. Also the majority of the studies utilised in this review were unadjusted for confounders known to affect survival of cancer patients such as age and stage [60, 61]. It is therefore difficult to determine how age and stage would affect the association of MAD2 expression on patient survival [62–64]. Of note, in the study that adjusted for age and stage the authors demonstrated that the association of MAD2 expression and survival was independent of these two confounders [25]. Therefore, future studies to analyse MAD2 IHC expression and survival should adjust for known cofounders to improve the reliability of results. Furthermore, as the studies examining MAD2 expression by IHC have utilised different sample numbers, power analysis should be considered before the study is undertaken to ensure that the data correctly identifies a statistical significant difference [65, 66]. Tissue microarrays may make a greater number of samples more achievable [67]. Despite these limitations, the overall outcomes remained consistent.
Recommendations for future studies evaluating the association of MAD2 IHC expression include standardising the reporting of results investigating high vs. low MAD2 expression (the cut-offs may differ between different tumour types), and include required statistical values in order to undertake a meta-analysis such as HR and 95% CI. Additional studies should adjust for known clinical prognostic factors such as age and stage and also carry out power analysis. In this review, apart from ovarian cancer, there has only been one study in each cancer type that has met the required statistical findings in order to be included in a meta-analysis (Table 2). Thus, more studies in each cancer type are required to definitively determine if the correlation of MAD2 IHC expression with patient survival is cancer-specific. Furthermore, investigation of the molecular pathways involving MAD2 is essential to help researchers understand the mechanism behind the results of this review. Recent studies have also highlighted a potential therapeutic role for MAD2 where silencing of the MAD2 gene using siRNA lead to increased apoptotic cell death in a non-small cell lung cancer model [68]. Additionally, MAD2's role as a predictive biomarker could also be particularly important. Further studies of cancer samples from patients treated with and without taxol/DNA- damaging agents would evaluate if MAD2 expression could predict the efficacy of these treatments on patient survival.
In conclusion, this review illustrates the potential for MAD2 IHC as a prognostic biomarker in cancer, especially in ovarian cancer where there is approximately a 50% lower risk of death or tumour recurrence in patients in the higher MAD2 expression category compared to the lower. An opposing prognostic role for increased risk of death in other cancer sites displaying high MAD2 expression may exist. Further studies are critical to assess the clinical utility of a MAD2 IHC biomarker and the molecular pathways affected by MAD2 that may improve patient outcomes. We outline recommendations for the standardised conduct and reporting of MAD2 expression studies in relation to cancer progression outcomes.
MATERIALS AND METHODS
This systematic review and meta-analysis adhered to the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [22].
Search strategy
Systematic literature searches were performed using three electronic databases; Medline (US National Library of Medicine, National Institutes of Health, Bethesda, Maryland, USA), Embase (Reed Elsevier PLC, Amsterdam, The Netherlands) and Web of Science (Thomson Reuters, New York, USA) from their origin until week five October 2015. A systematic search to identify publications, in any language, was applied using the following keywords and/or Medical Subject Heading (MeSH) terms; (Mitotic Arrest Deficient-Like 1 OR MAD2 or HSMAD2 or MAD2-Like Protein 1 OR Mitotic Arrest Deficient 2-Like Protein 1 OR Mitotic Spindle Assembly Checkpoint Protein MAD2A) AND (carcinoma(s) OR cancer(s) OR neoplasm(s) OR tumo (u) r(s) OR adenocarcinoma(s)).
Study selection
Inclusion criteria for studies were defined using the PICO model (population, intervention, control/comparison and outcome). The population included patients with any type of cancer. In order to be included in this systematic review, these studies needed to include the intervention which was an IHC method for the assessment of MAD2 expression. Studies must also have reported a comparison between high/positive and low/negative MAD2 levels in cancerous tissue. The outcome of the study must have reported either the proportion of tissue samples exhibiting MAD2 expression (or data from which this proportion could be calculated) and/or the statistical association between MAD2 and prognosis in cancer patients. Conference abstracts and poster presentations were excluded. Only human studies and literature available in the English language were included. Where publications reported on a sample source that was entirely overlapping and reported on the same outcomes, only the largest/most recent set of results was retained.
The principal reviewer (T. Byrne) screened all titles and abstracts, and two other reviewers (F. Furlong and J. Cooper) independently screened half of the titles and abstracts each. In cases where an abstract was unavailable or the article's significance was unclear, the full article was acquired for further examination. Those identified by any of the two reviewers for possible inclusion were brought forward for full text review.
Data extraction
From the full text of identified articles, where available, the following data was extracted by the principal reviewer (T.Byrne): authors, year of publication, journal name, location and number of centres samples were obtained from, primary aim of the study, source and method which samples were obtained, number of participants, mean age and sex of participants, immunohistochemical methods, type of antibody, manufacturer and dilution of antibody, type of staining, scoring system, IHC assessment procedure, number of patients with positive/negative/weak/moderate/strong MAD2 expression tumours, follow-up time, survival, and/or recurrence results including reported hazard ratios (HRs), confidence intervals (CIs) and associated p-values. Authors from publications that did not have all required information were contacted to obtain information and summary-level data was utilised for meta-analysis.
Statistical analysis
Meta-analyses were conducted to determine the association between MAD2 expression and overall survival and progression-free survival, where possible. Several of these studies did not present the results required for meta-analysis as high v. low comparisons of MAD2 expression. In the first instance authors were contacted to request information (provided by Furlong et al, Genga et al, Ko et al) [20, 23, 24]. For one study, Park et al, the HR for low v. high comparison was inverted in order to achieve comparability with studies where the HR for high v. low comparison was provided [25]. For other studies, the HR and 95% CI were derived by applying Parmar's methods [26] when the number of observed deaths in the high and low comparison groups had been presented together with the log-rank p-value for the associated Kaplan Meier curve (conducted for Li et al, Zhang et al, Nakano et al – serous and mucinous ovarian cancer studies, Suraokar et al, Kim, Choi, Yu, Gladhaug et al and Genga et al studies) [21, 23, 27–34]. Adjusted/unadjusted HRs and 95% CI (with the maximally adjusted rates used where possible) were combined and weighted to provide pooled estimates. A random effects model was utilised in order to account for the anticipated heterogeneity between studies. Subgroup analysis was possible by cancer type for ovarian cancer studies, where at least three studies had been published, but not for other cancer types. The extent of heterogeneity in the pooled estimates was calculated using the χ2 test and I2 statistic. The I2 statistic estimates the degree of variation between findings that is a result of heterogeneity rather than chance [35]. An I2 statistic above 25%, 50% or 75% was decided to denote mild, moderate and high heterogeneity respectively [36]. In order to evaluate publication bias, funnel plots were constructed according to the Begg and Egger methods, by plotting relative risks to their corresponding standard errors [37, 38]. In addition, sensitivity analysis was conducted to determine excessive influences of studies by removing each study individually and assessing the effect on pooled estimates and the heterogeneity I2 statistic [39]. Statistical analysis was conducted utilising Stata version 11.2 software (Stata Corporation, College Station, TX, USA).
SUPPLEMENTARY MATERIALS FIGURE AND TABLES
Abbreviations
- MAD2
mitotic arrest deficiency protein 2
- SAC
spindle assembly checkpoint
- BRCA1
breast cancer 1
- CHFR
Checkpoint with Forkhead And Ring Finger Domains, E3 Ubiquitin Protein Ligase
- CIs
confidence intervals
- E2F
E2 factor
- HRs
hazard ratios
- IHC
immunohistochemistry
- MeSH
Medical Subject Heading
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- TMAs
tissue microarrays
Footnotes
Authors’ contributions
TB, HC, JC, GMCC, AMcC and FF coneived the project and contributed to study design; TB, JC, FF performed data collection; TB, HC, JC and FF analysed and interpreted the data with HC undertaking the statistical analysis; TB wrote the manuscript with input from all authors; all authors read and approved the final version of the manuscript.
CONFLICTS OF INTEREST
No conflicts of interest exist for any author.
FUNDING
The project was funded by Department of Employment and Learning, Northern Ireland. No disclaimers.
REFERENCES
- 1.Mossaid I, Fahrenkrog B. Complex Commingling: Nucleoporins and the Spindle Assembly Checkpoint. Cells. 2015;4:706–25. doi: 10.3390/cells4040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sotillo R, Hernando E, Díaz-Rodríguez E, Teruya-Feldstein J, Cordón-Cardo C, Lowe SW, Benezra R. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schvartzman JM, Duijf PH, Sotillo R, Coker C, Benezra R. Mad2 is a critical mediator of the chromosome instability observed upon Rb and p53 pathway inhibition. Cancer Cell. 2011;19:701–14. doi: 10.1016/j.ccr.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabeche L, Compton DA. Checkpoint-independent stabilization of kinetochore-microtubule attachments by Mad2 in human cells. Curr Biol. 2012;22:638–44. doi: 10.1016/j.cub.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sotillo R, Schvartzman JM, Socci ND, Benezra R. Mad2-induced chromosome instability leads to lung tumour relapse after oncogene withdrawal. Nature. 2010;464:436–40. doi: 10.1038/nature08803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michel L, Benezra R, Diaz-Rodriguez E. MAD2 dependent mitotic checkpoint defects in tumorigenesis and tumor cell death: a double edged sword. Cell Cycle. 2004;3:990–92. [PubMed] [Google Scholar]
- 7.O’Neill TJ, Zhu Y, Gustafson TA. Interaction of MAD2 with the carboxyl terminus of the insulin receptor but not with the IGFIR. Evidence for release from the insulin receptor after activation. J Biol Chem. 1997;272:10035–40. doi: 10.1074/jbc.272.15.10035. [DOI] [PubMed] [Google Scholar]
- 8.Poelzl G, Kasai Y, Mochizuki N, Shaul PW, Brown M, Mendelsohn ME. Specific association of estrogen receptor beta with the cell cycle spindle assembly checkpoint protein, MAD2. Proc Natl Acad Sci USA. 2000;97:2836–39. doi: 10.1073/pnas.050580997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson KK, Schlöndorff J, Blobel CP. Evidence for an interaction of the metalloprotease-disintegrin tumour necrosis factor alpha convertase (TACE) with mitotic arrest deficient 2 (MAD2), and of the metalloprotease-disintegrin MDC9 with a novel MAD2-related protein, MAD2beta. Biochem J. 1999;343:673–80. [PMC free article] [PubMed] [Google Scholar]
- 10.Date DA, Burrows AC, Venere M, Jackson MW, Summers MK. Coordinated regulation of p31(Comet) and Mad2 expression is required for cellular proliferation. CellCycle. 2013;12:3824–32. doi: 10.4161/cc.26811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang RH, Yu H, Deng CX. A requirement for breast-cancer-associated gene 1 (BRCA1) in the spindle checkpoint. Proc Natl Acad Sci USA. 2004;101:17108–13. doi: 10.1073/pnas.0407585101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menssen A, Epanchintsev A, Lodygin D, Rezaei N, Jung P, Verdoodt B, Diebold J, Hermeking H. c-MYC delays prometaphase by direct transactivation of MAD2 and BubR1: identification of mechanisms underlying c-MYC-induced DNA damage and chromosomal instability. Cell Cycle. 2007;6:339–52. doi: 10.4161/cc.6.3.3808. [DOI] [PubMed] [Google Scholar]
- 13.Keller JA, Petty EM. CHFR binds to and regulates MAD2 in the spindle checkpoint through its cysteine-rich domain. Biochem Biophys Res Commun. 2011;409:389–93. doi: 10.1016/j.bbrc.2011.04.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernando E, Nahlé Z, Juan G, Diaz-Rodriguez E, Alaminos M, Hemann M, Michel L, Mittal V, Gerald W, Benezra R, Lowe SW, Cordon-Cardo C. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 15.Furlong F, Prencipe M, McGoldrick A, McGettigan P, Carney D, Doyle E, Kay E, McCann A. miR-433 overexpression attenuates the spindle assembly checkpoint response to paclitaxel. Breast Cancer Res. 2010;12:35. [Google Scholar]
- 16.Tambe M, Pruikkonen S, Mäki-Jouppila J, Chen P, Elgaaen BV, Straume AH, Huhtinen K, Cárpen O, Lønning PE, Davidson B, Hautaniemi S, Kallio MJ. Novel Mad2-targeting miR-493-3p controls mitotic fidelity and cancer cells’ sensitivity to paclitaxel. Oncotarget. 2016;7:12267–85. doi: 10.18632/oncotarget.7860. https://doi.org/10.18632/oncotarget.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li GQ, Li H, Zhang HF. Mad2 and p53 expression profiles in colorectal cancer and its clinical significance. World J Gastroenterol. 2003;9:1972–75. doi: 10.3748/wjg.v9.i9.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pati D, Haddad BR, Haegele A, Thompson H, Kittrell FS, Shepard A, Montagna C, Zhang N, Ge G, Otta SK, McCarthy M, Ullrich RL, Medina D. Hormone-induced chromosomal instability in p53-null mammary epithelium. Cancer Res. 2004;64:5608–16. doi: 10.1158/0008-5472.CAN-03-0629. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Yin F, Du Y, Du W, Chen B, Zhang Y, Wu K, Ding J, Liu J, Fan D. MAD2 as a key component of mitotic checkpoint: A probable prognostic factor for gastric cancer. Am J Clin Pathol. 2009;131:793–801. doi: 10.1309/AJCPBMHHD0HFCY8W. [DOI] [PubMed] [Google Scholar]
- 20.Furlong F, Fitzpatrick P, O’Toole S, Phelan S, McGrogan B, Maguire A, O’Grady A, Gallagher M, Prencipe M, McGoldrick A, McGettigan P, Brennan D, Sheils O, et al. Low MAD2 expression levels associate with reduced progression-free survival in patients with high-grade serous epithelial ovarian cancer. J Pathol. 2012;226:746–55. doi: 10.1002/path.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi JW, Kim Y, Lee JH, Kim YS. High expression of spindle assembly checkpoint proteins CDC20 and MAD2 is associated with poor prognosis in urothelial bladder cancer. Virchows Arch. 2013;463:681–87. doi: 10.1007/s00428-013-1473-6. [DOI] [PubMed] [Google Scholar]
- 22.Pérez de Castro I, de Cárcer G, Malumbres M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis. 2007;28:899–912. doi: 10.1093/carcin/bgm019. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira JH, Silva P, Faria J, Ferreira I, Duarte P, Delgado ML, Queirós O, Moreira R, Barbosa J, Lopes CA, do Amaral JB, Monteiro LS, Bousbaa H. Clinicopathologic significance of BubR1 and Mad2 overexpression in oral cancer. Oral Dis. 2015;21:713–20. doi: 10.1111/odi.12335. [DOI] [PubMed] [Google Scholar]
- 24.Bargiela-Iparraguirre J, Prado-Marchal L, Pajuelo-Lozano N, Jiménez B, Perona R, Sánchez-Pérez I. Mad2 and BubR1 modulates tumourigenesis and paclitaxel response in MKN45 gastric cancer cells. Cell Cycle. 2014;13:3590–601. doi: 10.4161/15384101.2014.962952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka K, Nishioka J, Kato K, Nakamura A, Mouri T, Miki C, Kusunoki M, Nobori T. Mitotic checkpoint protein hsMAD2 as a marker predicting liver metastasis of human gastric cancers. Jpn J Cancer Res. 2001;92:952–58. doi: 10.1111/j.1349-7006.2001.tb01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu L, Liu S, Guo W, Zhang B, Liang Y, Feng Q. Upregulation of Mad2 facilitates in vivo and in vitro osteosarcoma progression. Oncol Rep. 2012;28:2170–76. doi: 10.3892/or.2012.2032. [DOI] [PubMed] [Google Scholar]
- 27.Sudo T, Nitta M, Saya H, Ueno NT. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004;64:2502–08. doi: 10.1158/0008-5472.can-03-2013. [DOI] [PubMed] [Google Scholar]
- 28.Hao X, Zhou Z, Ye S, Zhou T, Lu Y, Ma D, Wang S. Effect of Mad2 on paclitaxel-induced cell death in ovarian cancer cells. J Huazhong Univ Sci Technolog Med Sci. 2010;30:620–25. doi: 10.1007/s11596-010-0553-y. [DOI] [PubMed] [Google Scholar]
- 29.Cheung HW, Jin DY, Ling MT, Wong YC, Wang Q, Tsao SW, Wang X. Mitotic arrest deficient 2 expression induces chemosensitization to a DNA-damaging agent, cisplatin, in nasopharyngeal carcinoma cells. Cancer Res. 2005;65:1450–58. doi: 10.1158/0008-5472.CAN-04-0567. [DOI] [PubMed] [Google Scholar]
- 30.Fung MK, Cheung HW, Wong HL, Yuen HF, Ling MT, Chan KW, Wong YC, Cheung AL, Wang X. MAD2 expression and its significance in mitotic checkpoint control in testicular germ cell tumour. Biochim Biophys Acta. 2007:821–32. doi: 10.1016/j.bbamcr.2007.03.014. 1773. [DOI] [PubMed] [Google Scholar]
- 31.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu L, Guo WC, Zhao SH, Tang J, Chen JL. Mitotic arrest defective protein 2 expression abnormality and its clinicopathologic significance in human osteosarcoma. APMIS. 2010;118:222–29. doi: 10.1111/j.1600-0463.2009.02583.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Yang D, Sun Y, Shmulevich I, Xue F, Sood AK, Zhang W. Differing clinical impact of BRCA1 and BRCA2 mutations in serous ovarian cancer. Pharmacogenomics. 2012;13:1523–35. doi: 10.2217/pgs.12.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fung MK, Cheung HW, Ling MT, Cheung AL, Wong YC, Wang X. Role of MEK/ERK pathway in the MAD2-mediated cisplatin sensitivity in testicular germ cell tumour cells. Br J Cancer. 2006;95:475–84. doi: 10.1038/sj.bjc.6603284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullan PB, Quinn JE, Harkin DP. The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene. 2006;25:5854–63. doi: 10.1038/sj.onc.1209872. [DOI] [PubMed] [Google Scholar]
- 36.O’Reilly EA, Sharma S, Harrison M, Weiner-Gorzel K, Kelly C, Maguire A, McCormack J, Conlon S, Aherne S, Beggan C, Bambury R, Dorsey T, Ambs S, et al. Low nuclear MAD2 expression is associated with of lack of response to chemotherapy and radiotherapy in oestrogen receptor negative breast cancer patients. Int J Surg. 2013;11:592. [Google Scholar]
- 37.Brown J, Frumovitz M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr Oncol Rep. 2014;16:389. doi: 10.1007/s11912-014-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putzke AP, Ventura AP, Bailey AM, Akture C, Opoku-Ansah J, Celiktaş M, Hwang MS, Darling DS, Coleman IM, Nelson PS, Nguyen HM, Corey E, Tewari M, et al. Metastatic progression of prostate cancer and e-cadherin regulation by zeb1 and SRC family kinases. Am J Pathol. 2011;179:400–10. doi: 10.1016/j.ajpath.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y, Choi JW, Lee JH, Kim YS. MAD2 and CDC20 are upregulated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the uterine cervix. Int J Gynecol Pathol. 2014;33:517–23. doi: 10.1097/PGP.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 40.Duncan TJ, Al-Attar A, Rolland P, Harper S, Spendlove I, Durrant LG. Cytoplasmic p27 expression is an independent prognostic factor in ovarian cancer. Int J Gynecol Pathol. 2010;29:8–18. doi: 10.1097/PGP.0b013e3181b64ec3. [DOI] [PubMed] [Google Scholar]
- 41.Li GQ, Zhang HF. Mad2 and p27 expression profiles in colorectal cancer and its clinical significance. World J Gastroenterol. 2004;10:3218–20. doi: 10.3748/wjg.v10.i21.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi SR, Shi Y, Taylor CR. Antigen retrieval immunohistochemistry: review and future prospects in research and diagnosis over two decades. J Histochem Cytochem. 2011;59:13–32. doi: 10.1369/jhc.2010.957191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor CR. Quantitative in situ proteomics; a proposed pathway for quantification of immunohistochemistry at the light-microscopic level. Cell Tissue Res. 2015;360:109–20. doi: 10.1007/s00441-014-2089-0. [DOI] [PubMed] [Google Scholar]
- 44.Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part I: basic concepts and first analyses. Br J Cancer. 2003;89:232–38. doi: 10.1038/sj.bjc.6601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan JK, Loizzi V, Lin YG, Osann K, Brewster WR, DiSaia PJ. Stages III and IV invasive epithelial ovarian carcinoma in younger versus older women: what prognostic factors are important? Obstet Gynecol. 2003;102:156–61. doi: 10.1016/s0029-7844(03)00399-5. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal R, Kaye SB. Prognostic factors in ovarian cancer: how close are we to a complete picture? Ann Oncol. 2005;16:4–6. doi: 10.1093/annonc/mdi104. [DOI] [PubMed] [Google Scholar]
- 47.Clark TG, Stewart ME, Altman DG, Gabra H, Smyth JF. A prognostic model for ovarian cancer. Br J Cancer. 2001;85:944–52. doi: 10.1054/bjoc.2001.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKay A, Donaleshen J, Helewa RM, Park J, Wirtzfeld D, Hochman D, Singh H, Turner D. Does young age influence the prognosis of colorectal cancer: a population-based analysis. World J Surg Oncol. 2014;12:370. doi: 10.1186/1477-7819-12-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park PE, Jeong JY, Kim SZ, Park JY. MAD2 Expression in Ovarian Carcinoma: Different Expression Patterns and Levels among Various Types of Ovarian Carcinoma and Its Prognostic Significance in High-Grade Serous Carcinoma. Korean J Pathol. 2013;47:418–25. doi: 10.4132/KoreanJPathol.2013.47.5.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kopans DB, Halpern E, Hulka CA. Statistical power in breast cancer screening trials and mortality reduction among women 40-49 years of age with particular emphasis on the National Breast Screening Study of Canada. Cancer. 1994;74:1196–203. doi: 10.1002/1097-0142(19940815)74:4<1196::aid-cncr2820740403>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 51.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59:990–96. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Torlakovic EE, Riddell R, Banerjee D, El-Zimaity H, Pilavdzic D, Dawe P, Magliocco A, Barnes P, Berendt R, Cook D, Gilks B, Williams G, Perez-Ordonez B, et al. Canadian Association of Pathologists-Association canadienne des pathologistes National Standards Committee Canadian Association of Pathologists-Association canadienne des pathologistes National Standards Committee/Immunohistochemistry: best practice recommendations for standardization of immunohistochemistry tests. Am J Clin Pathol. 2010;133:354–65. doi: 10.1309/AJCPDYZ1XMF4HJWK. [DOI] [PubMed] [Google Scholar]
- 53.Nascimento AV, Singh A, Bousbaa H, Ferreira D, Sarmento B, Amiji MM. Mad2 checkpoint gene silencing using epidermal growth factor receptor-targeted chitosan nanoparticles in non-small cell lung cancer model. Mol Pharm. 2014;11:3515–27. doi: 10.1021/mp5002894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Genga KR, Filho FD, Ferreira FV, de Sousa JC, Studart FS, Magalhães SM, Heredia FF, Pinheiro RF. Proteins of the mitotic checkpoint and spindle are related to chromosomal instability and unfavourable prognosis in patients with myelodysplastic syndrome. J Clin Pathol. 2015;68:381–87. doi: 10.1136/jclinpath-2014-202728. [DOI] [PubMed] [Google Scholar]
- 56.Ko YH, Roh JH, Son YI, Chung MK, Jang JY, Byun H, Baek CH, Jeong HS. Expression of mitotic checkpoint proteins BUB1B and MAD2L1 in salivary duct carcinomas. J Oral Pathol Med. 2010;39:349–55. doi: 10.1111/j.1600-0714.2009.00835.x. [DOI] [PubMed] [Google Scholar]
- 57.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 58.Li L, Xu DB, Zhao XL, Hao TY. Combination analysis of Bub1 and Mad2 expression in endometrial cancer: act as a prognostic factor in endometrial cancer. Arch Gynecol Obstet. 2013;288:155–65. doi: 10.1007/s00404-012-2706-7. [DOI] [PubMed] [Google Scholar]
- 59.Zhang SH, Xu AM, Chen XF, Li DH, Sun MP, Wang YJ. Clinicopathologic significance of mitotic arrest defective protein 2 overexpression in hepatocellular carcinoma. Hum Pathol. 2008;39:1827–34. doi: 10.1016/j.humpath.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Nakano Y, Sumi T, Teramae M, Morishita M, Fukuda T, Terada H, Yoshida H, Matsumoto Y, Yasui T, Ishiko O. Expression of the mitotic-arrest deficiency 2 is associated with chemotherapy resistance in ovarian serous adenocarcinoma. Oncol Rep. 2012;28:1200–04. doi: 10.3892/or.2012.1907. [DOI] [PubMed] [Google Scholar]
- 61.Nakano Y, Sumi T, Morishita M, Fukuda T, Nobeyama H, Yoshida H, Matsumoto Y, Yasui T, Ishiko O. Mitotic arrest deficiency 2 induces carcinogenesis in mucinous ovarian tumors. Oncol Lett. 2012;3:281–86. doi: 10.3892/ol.2011.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suraokar MB, Nunez MI, Diao L, Chow CW, Kim D, Behrens C, Lin H, Lee S, Raso G, Moran C, Rice D, Mehran R, Lee JJ, et al. Expression profiling stratifies mesothelioma tumors and signifies deregulation of spindle checkpoint pathway and microtubule network with therapeutic implications. Ann Oncol. 2014;25:1184–92. doi: 10.1093/annonc/mdu127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gladhaug IP, Westgaard A, Schjølberg AR, Burum-Auensen E, Pomianowska E, Clausen OP. Spindle proteins in resected pancreatic head adenocarcinomas: BubR1 is an independent prognostic factor in pancreatobiliary-type tumours. Histopathology. 2010;56:345–55. doi: 10.1111/j.1365-2559.2010.03489.x. [DOI] [PubMed] [Google Scholar]
- 64.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 65.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 67.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–57. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.