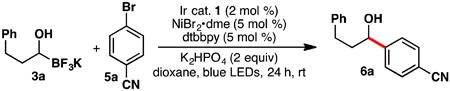
Table 1. Optimization of the Reaction Conditionsa.
| ||
|---|---|---|
|
| ||
| entry | deviation from standard condition | yield (%)b |
| 1 | no change | 66 |
| 2 | 5 mol % 4CzlPN instead of Ir cat. | 22 |
| 3 | 5 mol % Eosin Y instead of Ir cat. | 0 |
| 4 | 5 mol % MesAcr instead of Ir cat. | 0 |
| 5 | in absence of Ir cat. | 0 |
| 6 | in absence of nickel | 0 |
| 7 | in absence of light | 0 |
| 8 | in absence of K2HPO4 | 29 |
| 9 | DME instead of dioxane | 35 |
| 10 | MeCN instead of dioxane | 17 |
| 11 | DMF-df7 instead of dioxane | 32 |
|
| ||
| ||
General reaction conditions: Ar-Br (1.0 equiv, 0.1 mmol), trifluoroborate (1.3 equiv, 0.13 mmol), Ir catalyst (2 mol %), 1,4-dioxane (0.2 M), rt, 24 h.
Yield was determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
