Abstract
Objectives
Extraskeletal osteosarcoma (EO) is a malignant neoplasm that produces osteoid, bone, and chondroid material without direct attachment to bone or periosteum. Surgical resection is the mainstay of treatment; the role of chemotherapy is not well defined. Therefore, we evaluated the impact of chemotherapy in the survival of patients with EO.
Methods
All EO patients seen at Mayo Clinic between 1990 and 2014 were assessed. Forty-three patients were included after all archived pathology slides were reviewed to confirm the diagnosis of EO.
Results
Out of 43 patients, 37 patients had localized disease and 6 patients had metastatic disease at diagnosis. Chemotherapy was used in 73% and 75% of patients, respectively. Chemotherapy was predominantly anthracycline based, and included platinum in 22 patients (84%).
Median overall survival (OS) and progression-free survival (PFS) were 50 months (95% CI 25–99), and 21 months (95% CI 13-NR) respectively. There was a trend towards longer OS and PFS in patients who received chemotherapy. Those who received platinum-based therapy had remarkably prolonged OS (median 182 vs. 18 months; 5-year 61% vs 0% p=0.01) and PFS (median NR vs 10 months; 5-year 56% vs. 0%; p=0.005). Baseline characteristics were similar in the platinum and non-platinum group.
In patients who received chemotherapy, relapse/recurrence rate was lower in the platinum-based group (41%) as opposed to the non-platinum-based group (100%; p=0.02). In the neoadjuvant setting, the overall response rate of platinum-containing regimens was 27%.
Conclusions
Our results suggest a clinical benefit when platinum-based chemotherapy is incorporated in the management of patients with EO. We plan to validate this further with an expanded multi-center analysis.
Keywords: extraskeletal, extraosseous, osteosarcoma, chemotherapy, survival
INTRODUCTION
Extraskeletal osteosarcoma (EO) is a malignant mesenchymal neoplasm that produces osteoid, bone, and chondroid material without direct attachment to bone or periosteum. It is a rare malignancy, representing approximately 2 to 4% of the osteosarcomas1 and less than 1 % of the soft-tissue sarcomas.2,3 Extraskeletal osteosarcoma tends to present in an older age group, different anatomic location and has a higher recurrence rate and poorer survival than its osseous counterpart.4
Due to the rarity of extraskeletal osteosarcoma, formal randomized trials or prospective cohorts to define an optimal treatment strategy have not been feasible. Historically, patients who present with localized disease have been treated with surgical resection. Radiation is often employed for marginal or margin-positive resections. For systemic therapy, extrapolating the role of chemotherapy and identifying effective agents has been challenging. As such, chemotherapy has been sporadically utilized but remains controversial.5 A small number of studies have shown a trend toward improved outcomes with systemic chemotherapy. The chemotherapy regimens employed in patients with these tumors are typically those utilized for soft-tissue sarcomas5,6 rather than for skeletal osteosarcomas, which utilize platinum-based regimens. To our knowledge, no studies have compared outcomes of platinum-based systemic therapy with other agents.
We present our institution’s experience with extraskeletal osteosarcoma by describing clinical presentation, approach to treatment, and outcome analysis. We also aim to provide insight on optimal chemotherapy agents by comparing outcomes between platinum-containing chemotherapy and non-platinum-containing regimens.
METHODS
This study was approved by the Mayo Clinic Institutional Review Board. The Mayo Clinic, Rochester, MN electronic health records were queried to identify patients with the diagnosis of extraskeletal osteosarcoma seen from January 1, 1990 to December 31, 2014. Patients of all ages were included. Extraskeletal osteosarcoma was defined as an osteosarcoma without any involvement of bone or periosteum. Patients with prior history of skeletal osteosarcoma were excluded.
All available archived pathology slides were reviewed by a single bone and soft tissue pathologist to confirm the diagnosis. Histologic review of the tumors revealed pleomorphic cells with hyperchromatic nuclei admixed with variable amounts of neoplastic bone and osteoid matrix. The mitotic rates were generally brisk, and a subset of lesions contained necrosis. Fluorescence in situ hybridization (FISH) was used to detect amplification of Mouse double minute 2 homolog (MDM2) in all cases of retroperitoneal located tumors to rule out dedifferentiated liposarcoma.7 Patients with MDM2 amplification were excluded. The Federation Nationale des Centers de Lutte Contre le Cancer (FNFLFF) grading system was used for tumor grading. Grade 1 and 2 tumors were considered low grade while grade 3 tumors were considered high grade. Margin status at initial resection was defined as R0 if microscopically negative or R1 if microscopically positive. Primary tumor size was defined by maximum diameter measured by radiologic image before first radiation therapy or chemotherapy treatment or on the gross specimen when surgery was the first treatment modality. Tumor site was classified into upper extremity, lower extremity, axial (including chest wall, pelvis, perineum, head and neck) and retroperitoneal-visceral. Tumor depth was classified as superficial, deep (if deep to the fascia) or visceral. Radiologic reports including computed tomography or magnetic resonance imaging and biopsy report, when available, were used to confirm progression or recurrence. Neoadjuvant treatment response was assessed using the RECIST 1.1 criteria.8
STATISTICAL METHODS
Patient’s charts were retrospectively reviewed. Patient characteristics are summarized by frequency and percentage for categorical data and median with range for continuous data. Survival data are presented as median and 95% confidence interval and were calculated using the Kaplan-Meier method, compared using the log-rank test for univariate analysis. Overall survival was calculated from date of EO diagnosis to date of last follow-up. Patients who were alive at the last follow up were censored. Progression-free survival (PFS) data were calculated from the date of initial tumor resection to the date of first event (progression of disease or death). Patients without the event were censored for PFS at the last date they were found to be in remission and alive. Patients with metastatic disease at diagnosis who underwent surgical resection of all disease sites were included in the PFS analysis. Post relapse survival (PRS) data were calculated from the date of first progression/recurrence to the date of last follow up. For all tests, a p <0.05 was considered statistically significant. Analysis was conducted using JMP statistical software (SAS Institute Inc., Cary, NC).
RESULTS
PATIENT CHARACTERISTICS
Forty three patients with histologically confirmed EO were identified in the aforementioned 24-year time span. The patient and tumor characteristics are summarized in Table 1 and 2. The median follow up was 126 months (range 1.2 to 241 months). Two of 43 (5%) patients had history of prior radiation therapy to the site of EO at initial diagnosis. One patient received radiation to the pelvis for non-Hodgkin lymphoma 31 years before the diagnosis of EO of the hip. The other patient was diagnosed with a chest wall EO 28 years after receiving chest radiation therapy for metastatic testicular cancer. No cases of treatment-related myelodysplastic syndrome or acute leukemia were recorded.
Table 1.
Patient Demographics and Tumor Characteristics
| All patients (n=43) | |
|---|---|
| Age at diagnosis, median (range) | 55 (7–81) |
|
| |
| Gender* | |
| Male | 25 (58) |
|
| |
| Site of metastasis* | |
| At diagnosis (n=6) | |
| Lung | 4 (66) |
| Skin | 1 (17) |
| Heart | 1 (17) |
| At first relapse (n=18) | |
| Lung | 16 (89) |
| Liver | 1 (5) |
| Brain | 1 (5) |
| Musculoskeletal | 2 (11) |
|
| |
| Tumor Location* | |
| Upper extremity | 7 (16) |
| Lower Extremity | 19 (44) |
| Axial | 9 (21) |
| Retroperitoneal-visceral | 8 (19) |
|
| |
| Tumor Depth* | |
| Superficial | 11 (25) |
| Deep | 24 (56) |
| Visceral | 8 (19) |
|
| |
| Tumor Size* | |
| ≤ 5cm | 6 (15) |
| >5 cm and ≤ 10 cm | 19 (49) |
| >10 cm | 14 (36) |
|
| |
| Tumor Subtype* | |
| Osteoblastic | 26 (63) |
| Fibroblastic | 11 (27) |
| Chondroblastic | 4 (10) |
|
| |
| Recurrence* | |
| Local | 5 (13) |
| Distant | 17 (44) |
n (%) of patients with available data.
Table 2.
Tumor Characteristics by Frontline Chemotherapy Group
| Frontline chemotherapy
|
|||
|---|---|---|---|
| Platinum | Non-platinum | p value | |
| Tumor Location* | |||
| Upper extremity | 1 (5) | – | 0.18 |
| Lower Extremity | 15 (68) | 1 (25) | |
| Axial | 5 (23) | 1 (25) | |
| Retroperitoneal-visceral | 1 (5) | 2 (50) | |
|
| |||
| Tumor Depth* | |||
| Superficial | 5 (23) | 1 (25) | 0.06 |
| Deep | 16 (72) | 1 (25) | |
| Visceral | 1 (5) | 2 (50) | |
|
| |||
| Tumor Size* | |||
| ≤ 5cm | 4 (19) | 0.13 | |
| >5 cm and ≤ 10 cm | 10 (48) | 3 (100) | |
| >10 cm | 7 (33) | ||
|
| |||
| Tumor Subtype* | |||
| Osteoblastic | 14 (67) | 2 (67) | 0.33 |
| Fibroblastic | 6 (28) | 1 (33) | |
| Chondroblastic | 1 (5) | – | |
|
| |||
| Recurrence* | |||
| Local | 2 (10) | 2 (50) | 0.02 |
| Distant | 7 (32) | 2 (50) | |
|
| |||
| Metastatic at diagnosis* | 2 (10) | 1 (25) | 0.4 |
n (%) of patients with available data.
PATHOLOGY
The majority of tumors were greater than 5 cm (85%), with a median size of 8.7 cm (range 3.5 cm to 28.0 cm). Histologic review of all cases revealed malignant cells producing osteoid or bone matrix. A subset of cases (n=4) also exhibited significant malignant cartilage. All tumors showed marked cytologic atypia and were classified as high grade. FISH studies performed on all retroperitoneal located tumors (n=6) showed absence of MDM2 amplification in 4 patients. Two patients with MDM2 amplification were excluded prior to data analysis.
TREATMENT CHARACTERISTICS
Out of 43 patients, 37 (86%) had localized disease at diagnosis, of whom initial therapy information was available in 35 patients. All patients with localized disease underwent surgical resection. R0 margins were achieved in 73% of the available cases. Chemotherapy was used in 73% and radiation therapy in 69% of the patients. The remaining 6 patients (14%) had metastatic disease at the time of diagnosis and were treated with surgical resection (80%), chemotherapy (75%) and radiation therapy (60%). Surgical resection of all metastatic disease sites with curative intent was performed in 3 (50%) of these patients.
When considering all 43 patients, the initial treatment combinations were as follows: surgery, chemotherapy and radiation therapy (45%), surgery and chemotherapy (25%), surgery and radiation therapy (23%), surgery only (5%), radiation therapy only (2%). No patient received only chemotherapy as the initial therapy.
A total of 27 patients received chemotherapy as part of the initial treatment (including 3 patients with metastatic disease at diagnosis who underwent surgical resection with curative intent). Neoadjuvant chemotherapy was used in 16 (60%) patients, adjuvant chemotherapy was used in 9 patients (33%), while 2 patients (7%) received both neoadjuvant and adjuvant chemotherapy. At surgery, the median tumor necrosis was 80% [chemotherapy only, 57.5% (n=4); radiation therapy only, 42.5% (n=4); chemotherapy and radiation therapy, 90% (n=13)].
The most common frontline chemotherapy regimens were mitomycin/doxorubicin/cisplatin (MAP) (n=8, 30%), ifosfamide/mitomycin/doxorubicin/cisplatin (IMAP) followed by MAP (n=6, 22%), ifosfamide/doxorubicin (IA) (n=6, 22%) and IMAP (n=3, 11%). MAP was given concomitantly with radiation therapy in 12 (86%) of the patients. The typical cisplatin dose in the IMAP and MAP regimens was 60 mg/m2 or, when used in combination with radiation therapy, 45 mg/m2. All, but 1 patient, received doxorubicin as part of the first line chemotherapy regimen. Platinum-containing combination chemotherapy was used in 22 (84%) patients. The median number of chemotherapy cycles was 4 (range 2 to 6).
SURVIVAL CHARACTERISTICS
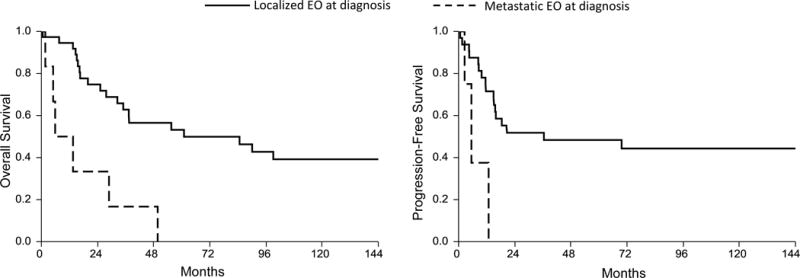
The median overall survival (OS) for this cohort was 50 months (95% CI 25–99), with a 5-year survival of 45% (Figure 1). The progression free survival (PFS) from surgical resection of the entire cohort was 21 months (95% CI 13-NR), with a 5-year PFS of 44% (Figure 1). The median post-relapse survival (PRS) was 13 months (95% CI 5–22). Six patients (14%) with metastatic disease at diagnosis (Table 1) had a significantly worse median OS compared to patients without metastatic disease (9.7 vs. 61 months respectively, p=0.0003).
Figure 1.
Overall Survival and Progression Free Survival for the entire cohort
Univariate analysis results are summarized on Table 3. Presence of metastases, large tumor size (>5 cm), advanced age (>55), necrosis rate (<95%), and deep or visceral tumor site all adversely impacted survival. There was no significant difference in survival based on tumor histological subtype, margin status at resection or patient gender.
Table 3.
Univariate Analysis of Factors Influencing Survival
| Variable | No. of patients | 5-Year Survival (%) | Hazard Ratio (95% CI) | Log-Rank p value |
|---|---|---|---|---|
| All patients | 43 | 45 | ||
|
| ||||
| Gender | 1.12 (0.5–2.6) | 0.76 | ||
| Female | 18 | 41 | ||
| Male | 25 | 44 | ||
|
| ||||
| Age (years) | 2.19 (1.0–4.9) | 0.04 | ||
| >55 | 21 | 30 | ||
| ≤55 | 22 | 59 | ||
|
| ||||
| Tumor depth* | 4.53 (1.4–15.6) | 0.01 | ||
| Visceral | 8 | 12 | ||
| Deep | 24 | 46 | ||
| Superficial | 11 | 68 | ||
|
| ||||
| Tumor location** | 3.34 (0.97–13) | 0.054 | ||
| Retroperitoneal-visceral | 8 | 13 | ||
| Axial | 9 | 42 | ||
| Lower Extremity | 19 | 54 | ||
| Upper extremity | 7 | 66 | ||
|
| ||||
| Size of primary tumor (cm)* | 5.85 (0.6–107) | 0.03 | ||
| >10 | 14 | 29 | ||
| 5–10 | 19 | 44 | ||
| <5 | 6 | 100 | ||
|
| ||||
| Margin Status at Resection | 1.82 (0.6–4.7) | 0.22 | ||
| R1 | 9 | 44 | ||
| R0 | 25 | 56 | ||
|
| ||||
| Metastatic at initial diagnosis | 5.0 (1.8–12) | 0.0003 | ||
| Yes | 6 | 0 | ||
| No | 37 | 53 | ||
|
| ||||
| Recurrence status | 7.3 (2.6–26) | 0.0001 | ||
| Recurrence (local and/or distant) | 20 | 20 | ||
| No recurrence | 19 | 79 | ||
Hazard ratio comparing first and third categories.
Hazard ratio comparing first and fourth categories.
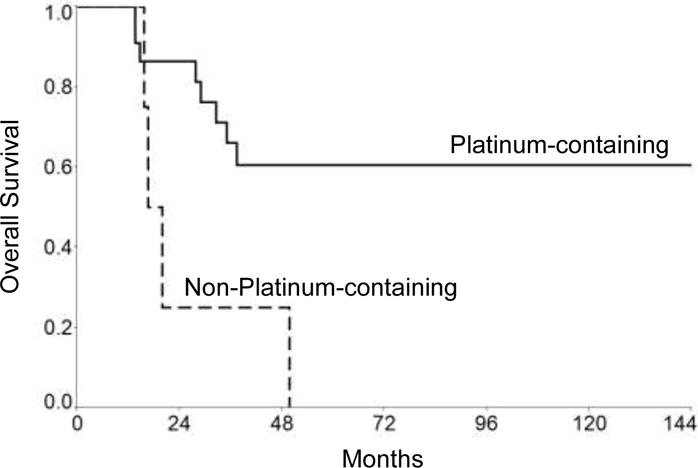
Table 4 shows survival characteristics based on initial treatment. Use of chemotherapy was associated with higher OS and PFS, although statistically significant only for patients treated with platinum-containing chemotherapy regimens (Table 4 and Figure 2). There was no significant difference in presence of metastasis at diagnosis, tumor size, age at diagnosis, or tumor location between patients who received platinum-containing chemotherapy compared to non-platinum-containing chemotherapy (Table 2). In the platinum-containing group, 9 patients (41%) had progressive/recurrent disease compared to all four patients in the non-platinum-containing group (p=0.02). However, the median post-relapse survival was similar in both groups [10 months (95% CI 2.7–21) vs 8.6 months (95% CI 0.9–45), p=0.74), respectively].
Table 4.
Survival Characteristics Based on Frontline Chemotherapy*
| Variable | No. of patients | 5-Year OS (%) | Median OS (95% CI), months | Log-Rank p |
|---|---|---|---|---|
| Chemotherapy, any regimen | 27 | 52 | 182 (29–NR) | 0.20 |
| No chemotherapy | 9 | 44 | 61 (5.8–99) | |
|
| ||||
| Platinum-containing chemotherapy | 22 | 61 | 182 (32-NR) | 0.01 |
| Other | 4 | 0 | 18 (16–50) | |
|
| ||||
| Variable | No. of patients | 5-Year PFS (%) | Median PFS (95% CI), months | Log-Rank p |
|
| ||||
| Chemotherapy, any regimen | 27 | 54 | 70 (12-NR) | 0.15 |
| No chemotherapy | 7 | 14 | 16 (0.6–36) | |
|
| ||||
| Platinum-containing chemotherapy | 22 | 62 | NR (13-NR) | 0.007 |
| Other | 4 | 0 | 10 (5.5–15) | |
Time to event analysis from initial diagnosis. Three patients with metastatic disease at diagnosis who underwent surgical resection of all disease sites with curative intent were also included in the OS and PFS analysis. OS - Overall Survival; PFS - progression free survival; NR - not reached
Figure 2.
Overall survival for Platinum-containing chemotherapy group
In the non-platinum group, 3 patients received IA (ifosfamide/doxorubicin) frontline chemotherapy, while the remaining patient received IE (ifosfamide/etoposide). All 4 patients underwent surgical resection. One patient also received radiation therapy. There were too few patients with metastatic disease at diagnosis for an accurate analysis of frontline therapy in OS and PFS.
Response rates from neoadjuvant chemotherapy were available in 11 patients, all of whom received platinum-containing chemotherapy. Complete response was seen in 1 patient (9%), partial response in 2 patients (18%) and stable disease in 8 patients (73%). No patients had progression of disease.
Of patients with available data, local recurrence was seen in 2 out of 14 patients (14%) who had radiation therapy compared to 3 out of 8 patients (37%) who did not received radiation therapy, p=0.30. Distant recurrence was seen in 11 of 25 patients (44%) who received chemotherapy compared to 5 out of 7 patients (71%) who did not received chemotherapy, p=0.39.
DISCUSSION
Extraskeletal osteosarcoma is a rare soft-tissue tumor associated with significant morbidity and mortality. Little is known regarding the best treatment approach for this specific cohort of patients. Even less is understood about the impact of systemic chemotherapy in the outcomes of patients with EO. The most common chemotherapy approach, as recommended by some authors,6,9 is to treat these patients as high-risk soft tissue sarcomas. This recommendation derived from analysis of response rates in a very small number of patients with EO treated with platinum-containing osteosarcoma regimens. Patel et al6 described 8 patients with EO treated with platinum (ORR 25%) compared to 5 patients who did not received platinum-containing regimens (ORR 20%). Ahmad et al9 reported a PR in 2 (13%) patients out of 15 treated with platinum compared to 2 (25%) patients out of 8 treated with other regimens. Others have suggested that a favorable outcome could be achieved when treating EO like conventional osteosarcoma.10 Chemotherapy however, has not been shown to convincingly impact overall survival in patients with localized soft tissue sarcoma, and remains a polarizing point of contention in the care of these patients.11 We therefore sought to elucidate the role of chemotherapy, and more specifically the impact of regimen choice in our institution’s large cohort of patients with extraskeletal osteosarcoma.
The median age at diagnosis of our cohort is similar to soft tissue sarcoma and previously reported series of EO,12–14 in stark contrast to osseous osteosarcoma, which commonly presents within the first 2 decades of life.15 Older age at diagnosis, tumor size and location were associated with worse prognosis, as described by previous reports.16 Metastatic disease at diagnosis and progression/recurrence, not surprisingly, are also poor prognostic markers.9,17 As described by Choi et al.,18 we also did not find an association of positive margins with worse survival, likely mitigated by the fact that 67% of these patients received radiation therapy. The 5-year OS and PFS seen in our study were similar to previously reported survival for soft tissue sarcoma,13 but inferior to osseous osteosarcomas.15
The conventional approach for patients with EO follows the principles established for patients with soft tissue sarcoma rather than primary osseous sarcoma. It is based on the combination of limb-sparing surgical resection, when possible, and radiation therapy. Chemotherapy is an acceptable option in the preoperative management for patients with locally advanced soft tissue sarcoma to aid operability as well as facilitate limb salvage. A retrospective review of 48 patients showed improved disease free survival with addition of adjuvant multiagent chemotherapy, but this result did not reach statistical significance.19 In our study, frontline chemotherapy was associated with improved outcomes without achieving statistical significance. The inability to detect a survival benefit in our study and others likely stems from unavoidable lack of power due to the rarity of this disease. Garnering multi-institution experiences with extraskeletal osteosarcoma may allow for better selection for patients who are likely to benefit from adjuvant chemotherapy.
Our cohort of extraskeletal osteosarcoma patients was also treated with a multimodality approach in most of the cases. All, but one patient underwent surgical resection of the primary tumor and the majority received radiation therapy. However, in contrast to the usual chemotherapy choice for soft-tissue sarcomas, a significant proportion of our patients received platinum-containing regimens.
Previous work by Ahmad et al.9 did not show a significant survival advantage with the use of chemotherapy, where 15 (25%) patients received platinum-containing therapy with an overall objective response (OOR) of 13% using a three-dimensional tumor volume response criteria. In our study, 22 (84%) patients received platinum-containing chemotherapy with an OOR of 27% using RECIST1.1 criteria. The use of platinum-containing chemotherapy in our patients significantly improved both PFS and OS compared to non-platinum-containing therapy (Table 4). The platinum-containing chemotherapy (IMAP and MAP) regimens during this time period were our standard chemotherapy regimen for soft tissue sarcoma. The platinum-containing regimens were not those of standard skeletal osteosarcoma and did not use high dose methotrexate.
It was not possible to assess the impact of surgical resection and radiation therapy in the survival of patients who presented with localized disease given that almost all patients underwent surgery and radiation therapy as part of their frontline therapy. All, but one patient, received doxorubicin in the first line of chemotherapy, which prevented any analysis regarding the impact of doxorubicin in the survival outcomes. Other limitations of our study include all inherent limitations of a retrospective review of a rare disease, including small number of patients and missing data with resulting limited analytic power. Even though our conclusions should be taken with reservation given the above stated limitations, this study represents one of the largest EO cohorts that focus on the impact of different chemotherapy regimens in this rare disease.
In conclusion, the results of our study suggest a survival benefit with the use of platinum-containing chemotherapy in the treatment of extraskeletal osteosarcoma. This contrasts with the historical treatment approach for extraskeletal osteosarcoma, but has also been proposed in the past by Goldestein-Jackson et al.10 Furthermore, a remarkable ORR of 27% was seen with preoperative use of platinum-containing regimens. The optimal treatment approach of patients with extraskeletal osteosarcoma is not well defined yet. Our study suggests consideration should be given to including perioperative platinum-containing chemotherapy in the management of these patients, akin to the conventional approach with osseous osteosarcoma. We are in the process of conducting a multi-institutional collaborative effort to further shed light on this provocative topic.
Acknowledgments
The work was funded in part by the National Institutes of Health (NIH) grant K12-CA090628.
Funding: The work was funded in part by the National Institutes of Health (NIH) grant K12-CA090628
References
- 1.Lorentzon R, Larsson SE, Boquist L. Extra-osseous osteosarcoma: a clinical and histopathological study of four cases. The Journal of bone and joint surgery British. 1979;61-B:205–8. doi: 10.1302/0301-620X.61B2.285933. [DOI] [PubMed] [Google Scholar]
- 2.Allan CJ, Soule EH. Osteogenic sarcoma of the somatic soft tissues. Clinicopathologic study of 26 cases and review of literature. Cancer. 1971;27:1121–33. doi: 10.1002/1097-0142(197105)27:5<1121::aid-cncr2820270519>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.McCarter MD, Lewis JJ, Antonescu CR, et al. Extraskeletal osteosarcoma: analysis of outcome of a rare neoplasm. Sarcoma. 2000;4:119–23. doi: 10.1080/13577140020008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lidang Jensen M, Schumacher B, Myhre Jensen O, et al. Extraskeletal osteosarcomas: a clinicopathologic study of 25 cases. The American journal of surgical pathology. 1998;22:588–94. doi: 10.1097/00000478-199805000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Lee MR, Lee SJ, et al. Extraosseous osteosarcoma: single institutional experience in Korea. Asia-Pacific journal of clinical oncology. 2010;6:126–9. doi: 10.1111/j.1743-7563.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- 6.Patel SR, Benjamin RS. Primary extraskeletal osteosarcoma–experience with chemotherapy. Journal of the National Cancer Institute. 1995;87:1331–3. doi: 10.1093/jnci/87.17.1331-a. [DOI] [PubMed] [Google Scholar]
- 7.Song MJ, Cho KJ, Lee JS, et al. Application of MDM2 Fluorescence In Situ Hybridization and Immunohistochemistry in Distinguishing Dedifferentiated Liposarcoma From Other High-grade Sarcomas. Appl Immunohistochem Mol Morphol. 2016 doi: 10.1097/PAI.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European journal of cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:521–7. doi: 10.1200/JCO.2002.20.2.521. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein-Jackson SY, Gosheger G, Delling G, et al. Extraskeletal osteosarcoma has a favourable prognosis when treated like conventional osteosarcoma. Journal of cancer research and clinical oncology. 2005;131:520–6. doi: 10.1007/s00432-005-0687-7. [DOI] [PubMed] [Google Scholar]
- 11.D’adamo D. Is adjuvant chemotherapy useful for soft-tissue sarcomas? The Lancet Oncology. 2012;13:968–970. doi: 10.1016/S1470-2045(12)70390-X. [DOI] [PubMed] [Google Scholar]
- 12.Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14:869–77. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 13.Pisters PW, Patel SR, Varma DG, et al. Preoperative chemotherapy for stage IIIB extremity soft tissue sarcoma: long-term results from a single institution. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15:3481–7. doi: 10.1200/JCO.1997.15.12.3481. [DOI] [PubMed] [Google Scholar]
- 14.Torigoe T, Yazawa Y, Takagi T, et al. Extraskeletal osteosarcoma in Japan: multiinstitutional study of 20 patients from the Japanese Musculoskeletal Oncology Group. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 2007;12:424–9. doi: 10.1007/s00776-007-1164-8. [DOI] [PubMed] [Google Scholar]
- 15.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thampi S, Matthay KK, Boscardin WJ, et al. Clinical Features and Outcomes Differ between Skeletal and Extraskeletal Osteosarcoma. Sarcoma. 2014;2014(902620) doi: 10.1155/2014/902620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mc Auley G, Jagannathan J, O’Regan K, et al. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR American journal of roentgenology. 2012;198:W31–7. doi: 10.2214/AJR.11.6927. [DOI] [PubMed] [Google Scholar]
- 18.Choi LE, Healey JH, Kuk D, et al. Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. The Journal of bone and joint surgery American. 2014;96:e2. doi: 10.2106/JBJS.M.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabbri N, Tiwari A, Umer M, et al. Extraskeletal osteosarcoma: Clinicopathologic features and results of multimodal management. Journal of Clinical Oncology. 2010;28 [Google Scholar]


