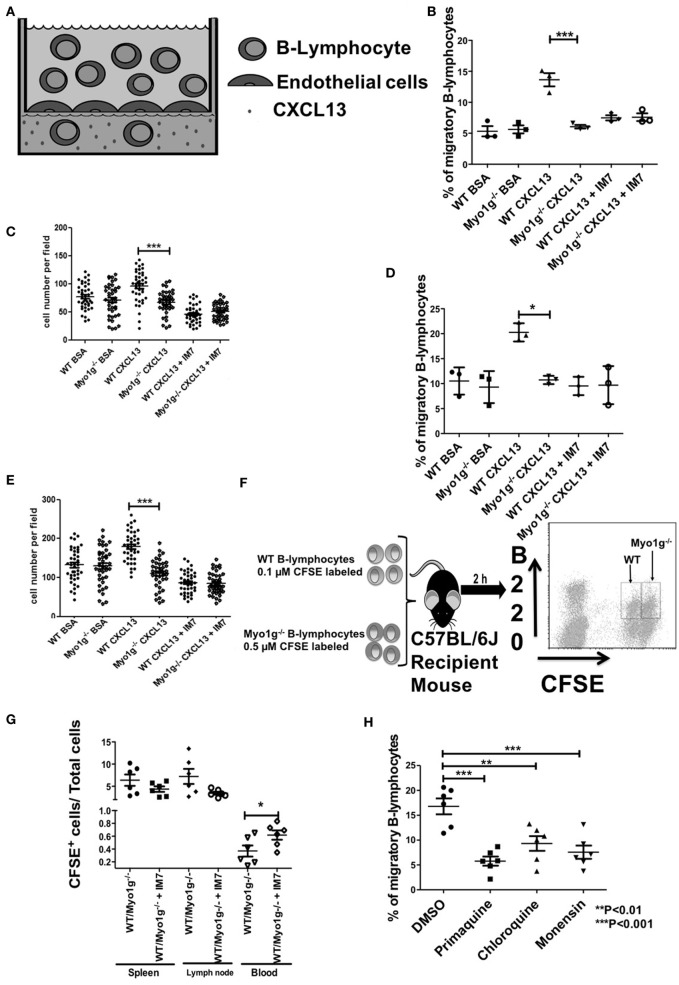
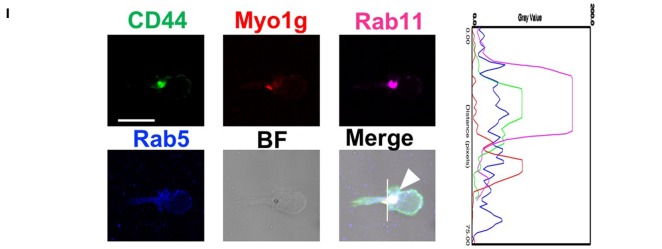
Figure 10.
Myo1g deficiency affects the migration of B lymphocytes. (A) Scheme of transendothelial migration where 4 × 105 B cells were placed in the upper compartment. (B,C) Analysis from resting WT or Myo1g-deficient B-lymphocytes. (D,E) Analysis of LPS plus IL-4 activated WT or Myo1g-deficient B lymphocytes. (B,E) Show the CXCL13-induced migration of B cells. (C,E) Percentage of migratory B cells that crossed the endothelial barrier. (F) Scheme of in vivo homing assay one-way ANOVA tests were used in these experiments, values are mean ± SD (*P < 0.05, ***P < 0.001) (n = 3). (G) 1 × 107 cells were transferred to recipient mice, the graph illustrates B cell recovery (CFSE+) percentage (WT/Myo1g−/−) from spleen, lymphatic nodes, and blood from Myo1g−/− and WT mice. Data are mean ± SD from three mice per assay in three independent experiments. One-way ANOVA test was used in these experiments, values are mean ± SD (*P < 0.05) (n = 6). (H) 4 × 105 resting B lymphocytes treated with vehicle, primaquine, chloroquine, or monensin were placed in the upper compartment during transendothelial transmigration. (I) Resting B-cells were placed on fibronectin-covered coverslips for 30 min, after that, the cells were treated with CXCL13 and incubated for 20 min, and then fixed and stained with antibodies for Rab5, Rab11, Myo1g, and CD44 for 20 min; the arrows indicate the location of these molecules in migratory B cells. The cells were then analyzed by confocal microscopy, scale bar 5 µm.