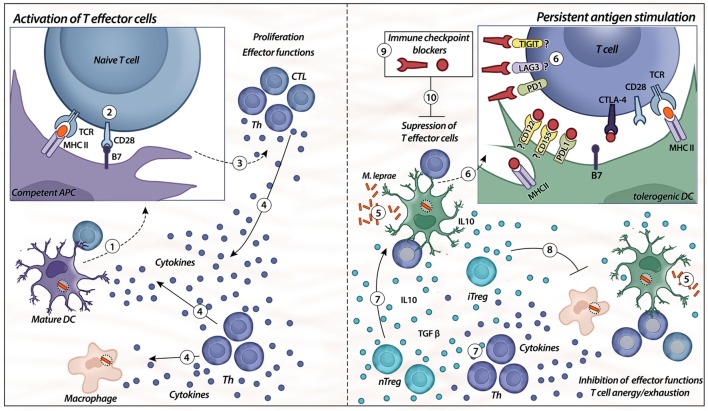
Figure 2.
Immune checkpoints. Activation of T effector cells is initiated with competent/mature antigen-presenting cells (APCs), such as mature dendritic cells (DC) (1, 2). For the first signal, APC displays the antigen to the naïve T cell via a complex with MHC II on their surfaces that is recognized by TCR on the surface of T cells; the second signal is nonspecific, resulting from the binding of B7 ligand on the APC with its receptor, CD28, on the T cell (2). When both signals are provided (3), T cells (different types of T helper and CTLs) exert their effector functions, such as release of cytokines by different Th cells (IL-6, IL-2, IFN-γ, IL-12, and TNF-α) and cytotoxicity from CTL (4). The presence of chronic immune stimulation due to persistent microbial antigens impairs specific cellular immunity (5, 6). Expression of co-inhibitory molecules, such as PD-1, TIGIT, lymphocyte-activation gene-3 (LAG-3), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), on lymphocytes and their respective ligands on the APC surface (PD-L1, CD122/155, MHC class II, and B7) induce specific T-cell anergy, leading to disseminated and progressive disease. In addition, there is higher differentiation of natural and induced types of Treg cells (nTreg/iTreg), as well as an imbalance of Th cells (7). The release of IL-10 and TGF-β from heterogeneous Treg cell subsets controls the immune response by the inhibition of effector functions, as well as induces tolerogenic phenotypes in DCs (8). The blockade of immune checkpoints, such as PD-1, CTLA-4, LAG-3, and TIGIT, might be a strategy to control the tolerogenic features observed in lepromatous leprosy patients (9, 10).