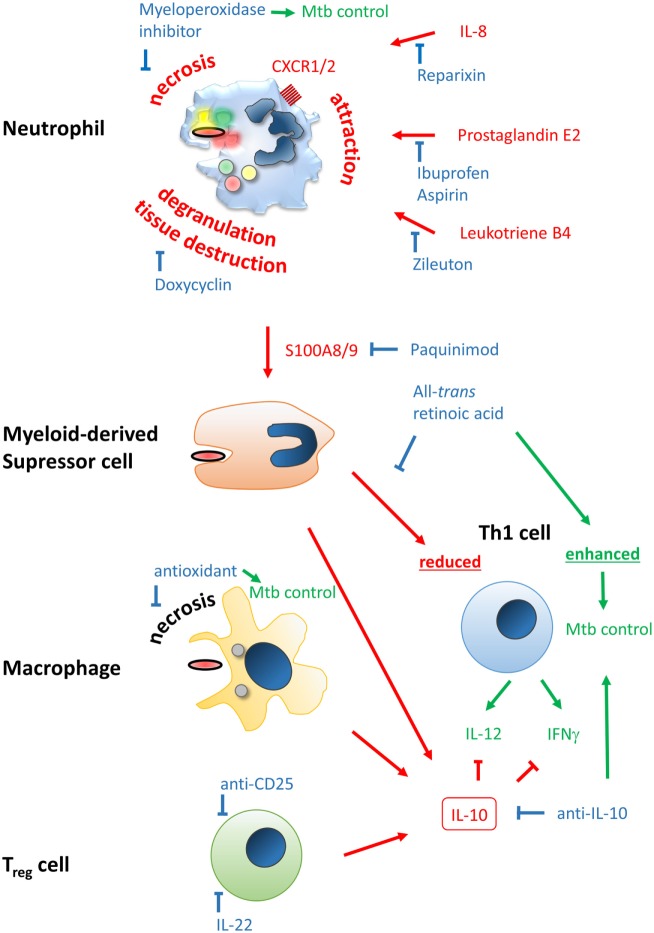
Figure 2.
Improving efficacy of classical BCG vaccines by host-directed immune modulation. Potentially detrimental molecules, mechanisms, and outcomes leading to poor vaccine efficacy are color-coded in red, beneficial ones in green, and modulators thereof in blue. Neutrophil infiltration at the site of vaccination may limit vaccine efficacy and generation of a proper and lasting Th1 memory response. Neutrophil attraction is mediated by IL-8, prostaglandin E2, and leukotriene B4, which can be blocked by Reparixin, Zileuton, and Ibuprofen/Aspirin, respectively. The type of cell death upon vaccination may be beneficial or detrimental, i.e., immunogenic apoptosis vs. necrosis, respectively. The latter can be blocked by the myeloperoxidase inhibitor ABAH or anti-oxidants. Degranulation of neutrophils’ highly toxic molecules, preventable by Doxycyclin, may lead to tissue destruction and release of the alarmin S100A8/9 that attracts myeloid-derived suppressor cells (MDSC). S100A8/9 can be blocked by Paquinimod. MDSC inherit highly potent T cell-suppressive properties, potentially limiting establishment of Th1-mediated immunity. This mechanism can be counteracted by all-trans retinoic acid. Among MDSC, macrophages and regulatory T cells may be attracted to the site of vaccination. Together they shift the immune response to an IL-10-driven Th2 phenotype that inhibits protective Th1 responses shaped by IL-12 and IFNγ, which have been shown to be protective against Mycobacterium tuberculosis infection. Spatiotemporal treatment with a blocking anti-IL-10 antibody may rescue successful generation of a Th1 immune signature. Application of rIL-22 or anti-CD25 antibody may limit regulatory T cell (Treg) contribution.