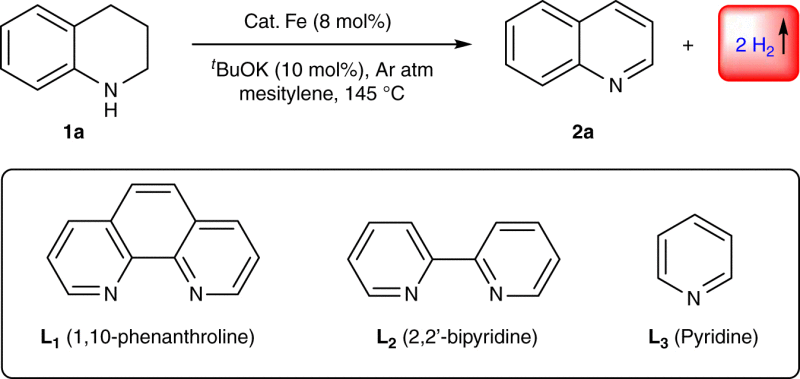
Table 1.
Acceptorless dehydrogenation of 1,2,3,4-tetrahydroquinoline (1a)
| |||
|---|---|---|---|
| Entry | Catalyst | Conversion (%)a | Yield (%)a |
| 1 | Fe-Phenb | Trace | Trace |
| 2 | Fe-Phen@EGOc | 20 | 16 |
| 3 | Fe@EGO | 17 | 15 |
| 4 | Phen@EGO | 6 | Trace |
| 5 | Fe-L1@EGO-400 | 40 | 37 |
| 6 | Fe-L1@EGO-600 | 52 | 45d |
| 7 | Fe-L1@EGO-900 | 98 | 92 (88)d |
| 8 | Fe-L2@EGO-900 | 51 | 40 |
| 9 | Fe-L3@EGO-900 | 30 | 22 |
| 10 | — | 0 | 0 |
| 11 | Fe-L1@Al2O3-900 | 23 | 19 |
| 12 | Fe-L1@SiO2-900 | 15 | 11 |
| 13 | Fe-L1@CeO2-900 | 8 | Trace |
| 14 | Fe-L1@TiO2-900 | 27 | 16 |
Reaction conditions: 1a (0.5 mmol), cat. Fe-L1@EGO-900 (8 mol%), t-BuOK (10 mol%), and mesitylene (2 mL) heated at 145 °C
aYields of 2a and conversion of 1a were determined by gas chromatography (GC)
bReaction under homogeneous conditions using the in situ-generated Fe catalyst
cNon-pyrolyzed materials
dIsolated yield
