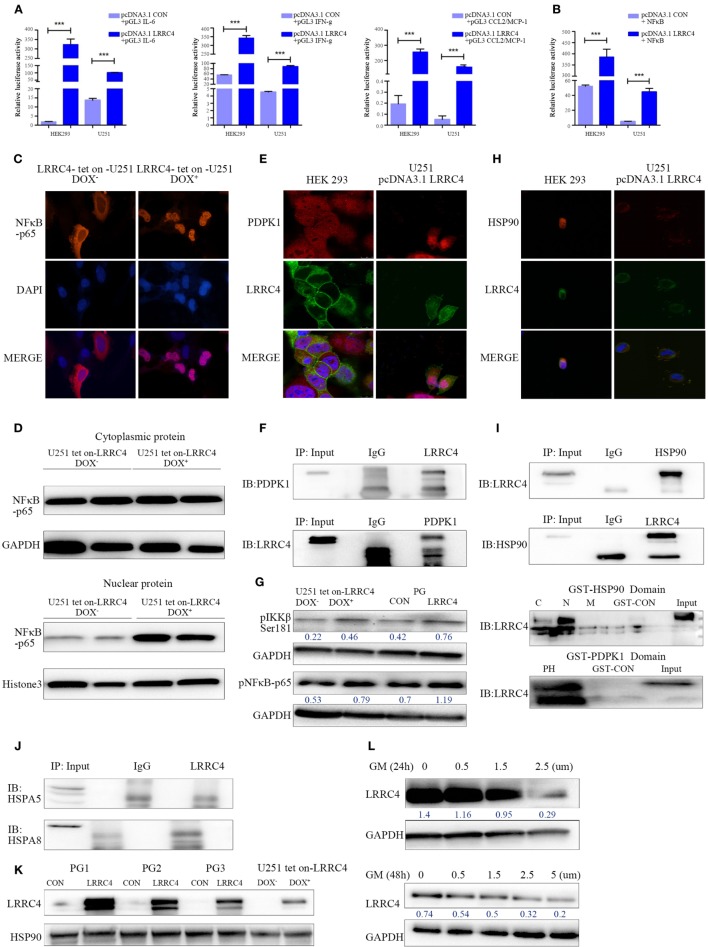
Figure 3.
LRRC4 facilitated IKKβ/NF-κB pathway activation by binding to phosphoinositide dependent protein kinase 1 (PDPK1), and HSP90 was required for the interaction of LRRC4 and PDPK1. (A,B) The Dual-Luciferase Reporter Assay indicated that LRRC4 promoted the transcriptional activity of interleukin-6 (IL-6) (***P < 0.001), interferon gamma (IFN-g) (***P < 0.001) and CCL2 [(A), P*** < 0.001], and induced NF-κB activation [(B), ***P < 0.001]. (C) Immunoflorescent staining for NF-κB-p65 revealed the nucleus translocation in U251 Tet-on-LRRC4 DOX+ cells compared with U251 Tet-on-LRRC4 DOX− cells. (D) The expression of NF-κB-p65 was mainly detected in the nucleus in U251 Tet-on-LRRC4 DOX+ cells. (E) Representative confocal and immunofluorescence images showing the co-localization of LRRC4 (green) and PDPK1 (red) in the cytoplasm in HEK293 and U251-pcDNA 3.1 LRRC4 cells. (F) Co-IP analysis showing the interaction between LRRC4 and PDPK1 in HEK293 and U251-pcDNA 3.1 LRRC4 cells. (G) Western blot analysis showing that the expression of pIKKβSer181 and pNF-κB p65 was increased in U251 Tet-on-LRRC4 DOX+ cells and PG-LRRC4 cells. (H) Representative confocal and immunofluorescence images showing the co-localization of LRRC4 (green) and HSP90 (red) in the cytoplasm in HEK293 and U251-pcDNA 3.1 LRRC4 cells. (I) Co-IP analysis showing the interaction between LRRC4 and HSP90 in U251-pcDNA 3.1 LRRC4 cells (up); GST pull-down assay showing that LRRC4 mainly bound to the N-domain of HSP90; LRRC4 mainly bound to the PH domain of PDPK1 (down). (J) Co-IP analysis showing no interaction between LRRC4 and HSPA5 and HSPA8 in U251-pcDNA 3.1 LRRC4 cells. (K) Western blot analysis of the levels of HSP90 showing no alteration in U251 Tet-on-LRRC4 DOX cells and PG-CON/LRRC4 cells. (L) The expression of LRRC4 was decreased when HSP90 activity was inhibited. The U251-pcDNA 3.1 LRRC4 cells were treated with selected concentrations and durations of the HSP90 inhibitor geldanamycin.