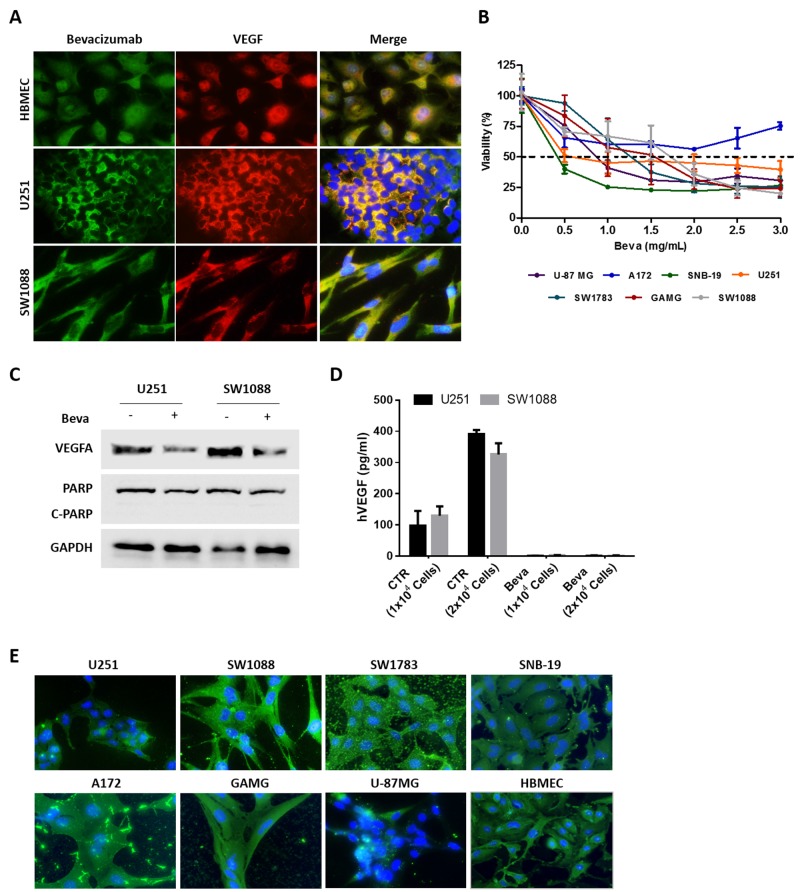
Figure 1. In vitro effect of Bevacizumab in GBM cell lines.
(A) Immunofluorescence for VEGFA using a specific anti-VEGF antibody and Beva as primary antibodies. (B) Cell viability of GBM cell lines exposed to increasing concentrations of Beva was assessed by MTS assay at 72 hours of treatment; results are from three independent assays, each one in triplicates. (C) Western Blot analysis for VEGF and the apoptotic marker (PARP cleavage). The cells were treated with 2 mg/ml of Beva during 24 hours. (D) ELISA assay for human VEGF. The cells were seeded in different numbers and the supernatant of the cells was collected after 24 hours of Beva treatment (2 mg/ml) for VEGF quantification. (E) Beva internalization was evaluated in GBM cell lines treated during 24 hours with Beva (2 mg/ml) by using an anti-human IgG antibody. On the images from (A) and (E) the cell nucleus were counterstained with DAPI and the pictures were taken at 400x in an Olympus fluorescence microscope.