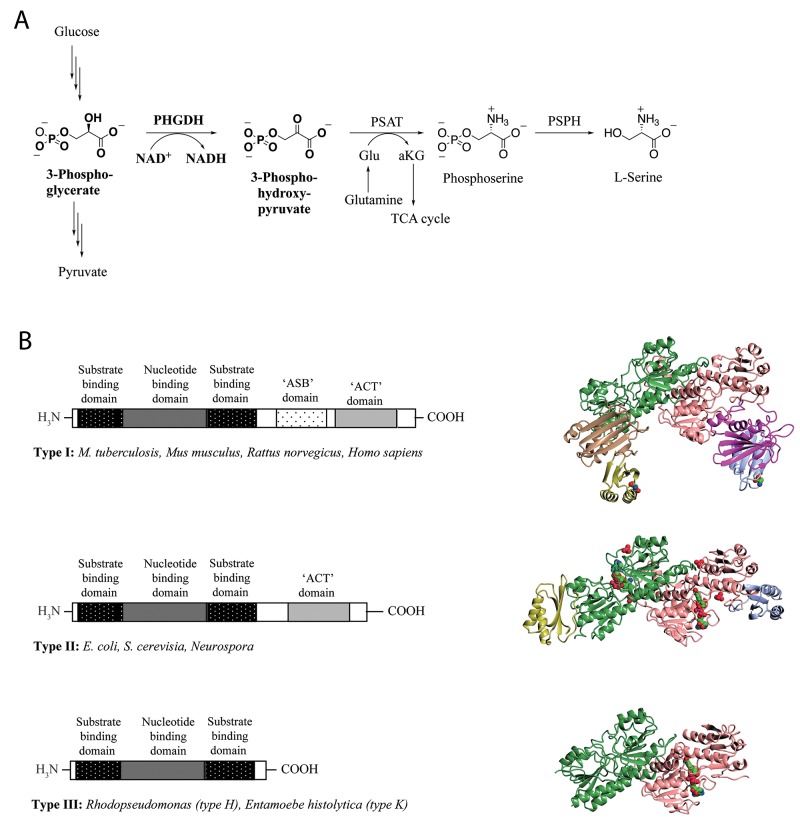
Figure 1. L-Serine synthesis pathway and basic domain structure of PHGDH.
(A) 3-Step synthesis scheme of endogenous L-serine starts with the oxidation of 3-phosphoglycerate to 3-phosphohydroxypyruvate by PHGDH and simultaneous reduction of the cofactor NAD+ to NADH. The subsequent transamination reaction is catalyzed by phosphoserine aminotransferase (PSAT), which uses glutamate (Glu) as nitrogen donor and thereby produces phosphoserine and α-ketoglutarate (αKG). Dephosphorylation of phosphoserine by phosphoserine phosphatase (PSPH) gives rise to L-serine. (B) Basic domain structure found within the three enzyme types of PHGDH shaded by domain. Additional amino acids at the N-terminus are not explicitly shown as variations in length and composition of this part of the protein depend on the species. Two forms of the type III enzyme exist depending on whether lysine (type K) or histidine (type H) is present at the active site [8] (left). Crystal structures of representative family members of the different types of PHGDH. For a better comparison, all enzymes are shown as dimers, although active PHGDH from M. tuberculosis and E. coli form a tetramer. The substrate- and nucleotide-binding domain is shown in green/rose, the ASB domain is shown in magenta/brown and the ACT domain is shown in blue/yellow. If present in the crystal, the cofactor NAD+ is depicted in spheres and colored by atom type (carbon in green) (right).