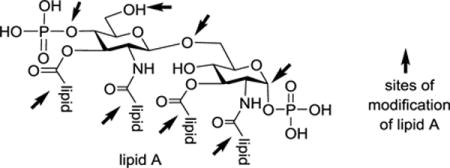
Table 1.
Natural lipid As and various analogs and derivatives of lipid As achieved by chemical synthesis
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Compound | Bacterial origin | Group that realized the synthesis | Year | Reference | Key structural and synthetic features | Biological outcomes |
| Natural lipid As and lipid A derivatives bearing saturated lipids and both phosphate groups | ||||||
| 1 | E. coli | Boons | 2007 | 43 | Natural lipid A with six acyl chains; synthesis based on an orthogonally protected disaccharide | Lower potency to induce cytokine production than E. coli LPS, suggesting the role of Kdo residues in LPS |
| 10 | S. typhimurium | Boons | 2007 | 43 | Natural lipid A with seven acyl chains. | Significantly lower potency to induce cytokine production than 1 |
| 11 | E. coli | Boons | 2007 | 43 | A lipid A derivative with shortened lipids | Higher potency to induce cytokine production than 1 |
| 12 | S. typhimurium | Boons | 2007 | 43 | A lipid A derivative with shortened lipids | Higher potency to induce cytokine production than 10 |
| 13 | S. typhimurium | Boons | 2007 | 43 | A lipid A derivative devoid of the anomeric phosphate group | Lower potency to induce cytokine production than 12 |
|
|
||||||
| 1 and 10–13 were synthesized by a similar strategy; their difference in biological activities indicated the importance of the number and length of the fatty acid lipid chains in lipid A | ||||||
|
|
||||||
| 26 | Ru. gelatinosus | Fukase | 2008 | 45 | Natural lipid A with six acyl chains; synthesis by using lapidated monosaccharide blocks | Potent ability to antagonize LPS endotoxic activity. |
| 27, 28 | C. trachomatis | Kosma | 2004 | 54 | C. trachomatis lipid A with tetra- or pentaacyl lipid chains. | |
|
| ||||||
| Natural lipid As and lipid A derivatives bearing unsaturated lipids | ||||||
| 34a,b | R. sphaeroides | Christ | 1994 | 37, 55, 56 | A derivative of natural lipid A; use of Alloc and All groups to protect the hydroxyl and phosphate groups | Devoid of LPS agonistic properties; strong in vitro antagonistic activity; suppress TNF-α production induced by LPS |
|
40 (E5531) |
R. sphaeroides | Yamatsu | 1995 | 57–59 | A derivative of natural lipid A with 3,3′-O-acyl groups substituted for hydrolytically stable ether linkages and C-6′-hydroxyl group blocked as methyl ether | Strong antagonist of cytokine release of induced by LPSs; completely devoid of LPS agonistic activity; protecting BCG-primed mice from LPS-induced lethality and E. coli infection-caused death. |
|
41 (E5564) |
R. sphaeroides | Christ | 2002 | 60–62 | Second generation of the above lipid derivatives | Excellent antagonist activity; clinical use in the trade name of Eritoran for the treatment of severe sepsis |
|
| ||||||
| Unnatural lipid A derivatives bearing two phosphate groups | ||||||
| 42–44 | artificial | Savage | 2003 | 40 | Containing four lipid chains with incrementally shortened length | Different solubility in water; used to study the interaction of lipid A with polymyxin B |
| 54–56 | E. coli | Kusama | 1990 | 69 | Lipid A derivatives containing anomeric α/β-phosphonooxyethyl group instead of labile α-glycosylphosphate. | α-glycosides had comparable antitumor activity as natural lipid A; β-glycoside was less active |
| 64, 65 | E. coli | Kusama | 1991 | 74 | Derivatives of lipid A biosynthetic precursor; containing anomeric α-phosphonooxyethyl | The first generation of potent antitumor lipid A derivatives with enhanced stability and reduced toxicity. |
| 66, 67 | E. coli | Fukase | 2001 | 75, 76 | Lipid A derivatives with tritium-labeled α-phosphonooxyethyl | Useful for the study of the interactions between lipid A and their acceptors |
|
| ||||||
| Lipid A analogs carrying an anomeric carboxylic acid | ||||||
| 74a–c, 75a–c | E. coli | Shiozaki | 2000 | 79, 80 | Lipid A derivatives with a carboxylic acid moiety in place of the glycosyl phosphate; 74a–c and 75a–c had six and four lipid chains; different 6′-O-substituents | 74a–c was a LPS agonist but 75a–c was a LPS antagonist, indicating the importance of the number of lipid chains; the carboxyl group and the 6′-O-substituent did not have a significant impact |
| 92a–c, 93a–c, 94a–c | artificial | Shiozaki | 2001 | 81 | Anomeric carboxylic acid; unnatural 3,3′-O-ether linkage; different 6′-C-substituents; 92 and 94 had four lipid chains; 93 had six | Improved stability; 92 and 94 were strong LPS antagonists; 93b was inactive; 93a,c were moderate LPS agonists; ether linkage and anomeric carboxylic acid did not have a major impact |
| 104a–c | artificial | Shiozaki | 2003 | 51 | Anomeric carboxymethyl group and 3,3′-O-ether linkages. | Improved stability; LPS antagonists; ether linkage and anomeric carboxylic acid did not have a major impact |
| 111a,b–116a,b | artificial | Fukase | 2006 | 84 | Monosaccharide analogs of lipid A with aspartic acid or phosphoserine in place of non-reducing end phosphorylated glucosamine unit | Most compounds except 115a,b showed antagonistic activity |
|
| ||||||
| Monophosphoryl lipid A and related derivatives | ||||||
| 119–121 | S. minnesota | Jiang | 2007 | 50 | S. minnesota R595 MPLA derivatives; different substituents at the 3-O-position | All were strong adjuvants; 121 was not more toxic than natural R595 MPLA 118 that devoid of 3-O-acyl chain |
| 127a-f | S. minnesota | Johnson | 1999 | 52 | S. minnesota R595 MPLA derivatives with variant fatty acids | Fatty acid chain length was important for inducing proinflammatory cytokines |
| 133–140 | E. coli K4 | Corsaro | 2016 | 94 | Semi-synthetic lipid A analogs derived from selective modification of the C-6′-OH, lipid pattern and phosphate | Some of them, especially 136, showed promising immunoadjuvant activity |
| 146 | N. meningitidis | Guo | 2009 | 95 | MPLA derivative with an amino group at the reducing end, readied for coupling with other carbohydrate antigens | Useful as a vaccine carrier for the development of self-adjuvant conjugate vaccines |
| 147–150 | N. meningitidis | Guo | 2014 | 96 | MPLA derivative with varied acyl chains with an amino group at the reducing end. | Used to discover new and optimized vaccine carriers and adjuvants. |
| 156 | E. coli | Guo | 2010 | 46 | MPLA derivative with an alkyne group at the reducing end for click reaction | Useful as a vaccine carrier for the development of self-adjuvant conjugate vaccines |
| 157–160 | P. gingivalis | Ogawa, Boons | 2007, 2008 | 99, 100 | Tetra-acylated MPLAs with branched lipid chains and devoid of 4′-O-phosphate | 159 and 160 showed potent antagonist activity, which was determined by their acylation pattern. |
|
| ||||||
| Non-phosphorylated lipid As and derivatives | ||||||
| 161–164 | R. sin-1 | Carlson, Vandenplas | 2002 | 101, 102 | Lack of both phosphate groups; aminogluconate in place of the reducing end glucosamine unit | Inhibited TNF-α release |
| 165, 166 | R. sin-1 | Boons | 2003 | 41 | Reducing end anomeric carbon had different oxidation states | The gluconolactone unit in 166 was important for its antagonistic activity. |
| 173, 174 | R. sin-1 | Boons | 2004 | 42 | Devoid of 3-O-acylation | Antagonists of LPS but less potent than 166, indicating the biological importance of 3-O-acyl chain |
| 188, 189 | R. sin-1 | Boons | 2007 | 44 | 189 contained the 27-hydroxyoctacosanoic group at its 2′-N-position | Lipid chain length, not the OH group on it, was critical for its antagonistic activity |
|
| ||||||
| LPS analogs and Kdo-lipid A conjugates | ||||||
|
199 (ReLPS) |
E. coli mutant | Kusumoto | 2001 | 53, 110 | First chemical synthesis of a short LPS with two Kdo units linked to lipid A; Kdo fluorides with bulky 4,5-O-protecting groups were used for α-glycosylation | 199 showed slightly less potent cytokine-inducing activity than that of the natural ReLPS |
| 212 | H. pylori | Fukase. | 2007 | 107 | Lipid A-Kdo conjugate with fewer but longer acyl chains; devoid of 4-O′-phosphate; Kdo fluorides was used for α-glycosylation | TLR-4 antagonists; Kdo residue could enhance the inhibitory activity |
| 221 | H. pylori | Fukase, Fujimoto | 2011 | 114 | Lipid A-Kdo conjugate with an ethanolamine unit in its structure; Kdo N-phenyltrifluoroacetimidate and a microfluidic glycosylation protocol was developed and used for α-glycosylation | Inhibition of cytokine production; the presence of a Kdo unit in the structure reversed the bioactivity of its parent lipid A 220 |
