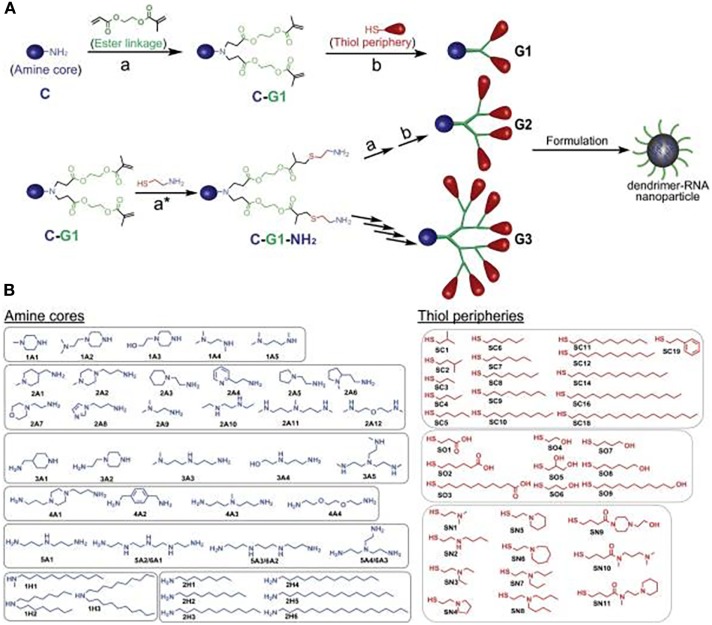
Figure 3.
A modular strategy for diversifying the chemical functionality and size of ester-based dendrimers allowed discovery of potent and nontoxic dendrimers for in vivo small-RNA delivery to tumor cells. (A) Orthogonal reactions accelerated the synthesis of >1,500 modular degradable dendrimers by combination of 42 cores (C) and 36 peripheries (P) through degradable linkages (L) and generations. The library was established via sequential reactions. First, amines (C) with a series of N–H bonds reacted quantitatively and selectively with the less steric acrylate groups of AEMA (L). The products (C–L) then quantitatively reacted with various thiols (P) under optimized DMPP-catalyzed conditions. (B) Dendrimers were independently modulated with chemically diverse amines and thiols. Selected amines were divided into two categories: ionizable amines (1A–6A) to tune RNA binding from C that generated one to six branched dendrimers, and alkyl amines (1H–2H) to tune NP C stabilization. Alkyl thiols (SC1–SC19) and alcohol/carboxylic acid terminated thiols (SO1–SO9) were selected to tune NP P stabilization. Aminothiols (SN1–SN11) were selected to tune P RNA binding. G2–G4 higher generation dendrimers with multiple branches were also synthesized using generation expansion reactions. Reproduced from Ref. (24) with kind permission by the National Academy of Sciences.