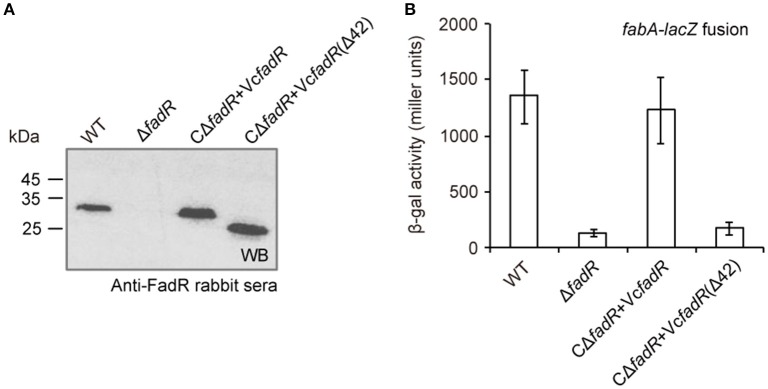
Figure 8.
FadR activates expression of fabA, an important gene for unsaturated fatty acid synthesis in V. cholerae. (A) Western blot analyses for fadR expression and its derivatives. The primary antibody used here is rabbit sera against V. cholerae FadR. (B) The beta-gal activity of fabA-lacZ transcription fusion is significantly decreased in the ΔfadR mutant, and can be restored by the functional complementation of the wild-type fadR [but not VcfadR(Δ42)]. The fadR deletion mutant strain referred to ΔfadR, and the two types of complemented strain of ΔfadR used corresponded to CΔfadR + VcfadR and CΔfadR + VcfadR(Δ42), respectively. The VcfadR(Δ42) denoted an engineered version of V. cholerae fadR with a 42-residue deletion covering the site 2 for ligand binding. Log-phase cultures were collected for assaying the LacZ activity. The β-gal activity is expressed as the mean ± standard deviation and is collected from no less than three independent experiments.