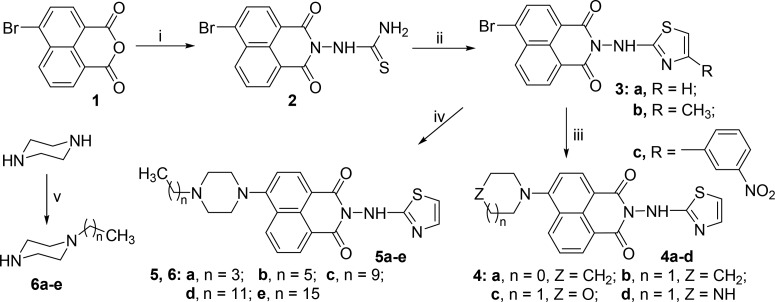
Scheme 1. Synthetic Route of Naphthalimide Aminothiazoles 3–5.
Reagents and conditions: (i) thiosemicarbazide, DMF, 100 °C, 8 h; (ii) α-halogenated carbonyl compounds, ethanol, 80 °C, 7 h; (iii) alicyclic amines, 2-methoxyethanol, reflux, N2, 7 h; (iv) N-alkylated piperazines, 2-methoxyethanol, reflux, N2, 7 h; (v) alkyl halides, ethanol, 80 °C, 8 h.