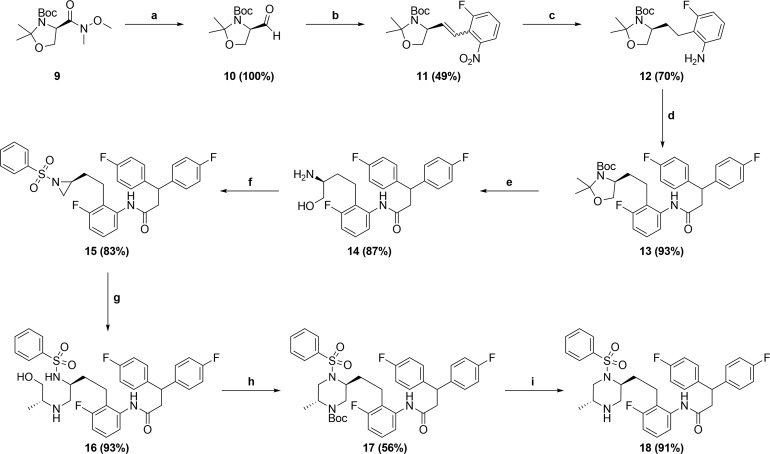
Scheme 2.
Reagents and conditions: (a) LiAlH4, 2-Me-THF, 0 °C; (b) K2CO3, 18-crown-6, (2-nitrobenzyl)triphenylphosphonium bromide, DME, RT; (c) Pearlman’s catalyst, 50 psi H2, EtOAc/MeOH, RT; (d) 3,3-Bis(4-fluorophenyl)propanoic acid, T3P, Hunig’s base, EtOAc, RT; (e) TFA, H2O, CH2Cl2; RT; (f) (i) PhSO2Cl, NEt3, DMF, 0 °C; (ii) diazene-1,2-diylbismorpholinomethanone, PBu3, THF, RT; (g) (R)-2-aminopropan-1-ol, 1,2-DCE, 40 °C; (h) (i) Boc2O, NEt3, CH2Cl2, RT; (ii) diazene-1,2-diylbismorpholinomethanone, PBu3, THF, RT; (i) TFA, CH2Cl2, RT.