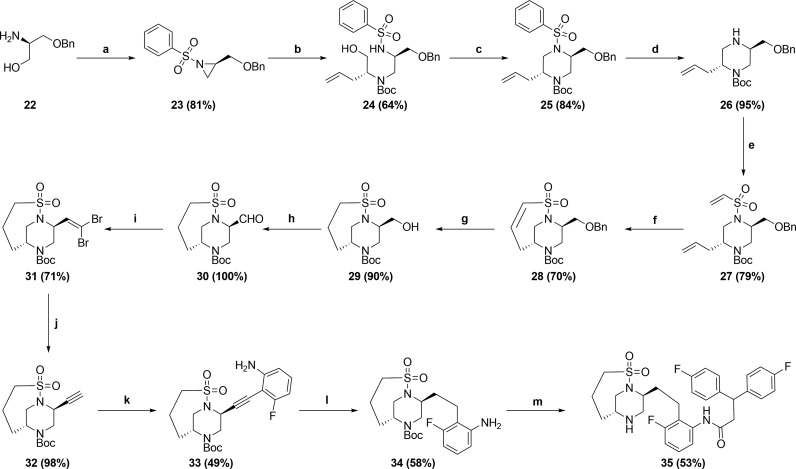
Scheme 3.
(a) (i) PhSO2Cl, NEt3, DMF, 0 °C; (ii) DIAD, PBu3, THF, 0 °C; (b) (i) (R)-2-aminopent-4-en-1-ol, THF, 45 °C; (ii) Boc2O, NEt3, CH3CN, 45 °C; (c) DIAD, PBu3, THF, RT; (d) Mg, MeOH, sonication, RT; (e) 2-chloroethanesulfonyl chloride, NEt3, CH2Cl2, RT; (f) Zhan Catalyst-1B, 1,2-DCE, 50 °C; (g) Pearlman’s catalyst, H2 balloon, EtOAc, RT; (h) Dess–Martin periodinane, CH2Cl2, RT; (i) PPh3, CBr4, CH2Cl2, RT; (j) EtMgBr, THF, 0 °C; (k) 3-fluoro-2-iodoaniline, (PPh3)2PdCl2, CuI, NEt3, CH3CN, 70 °C; (l) Pearlman’s catalyst, H2 balloon, EtOH, RT; (m) (i) 3,3-Bis(4-fluorophenyl)propanoic acid, T3P, Hunig’s base, EtOAc, RT; (ii) HCl, dioxane, RT.