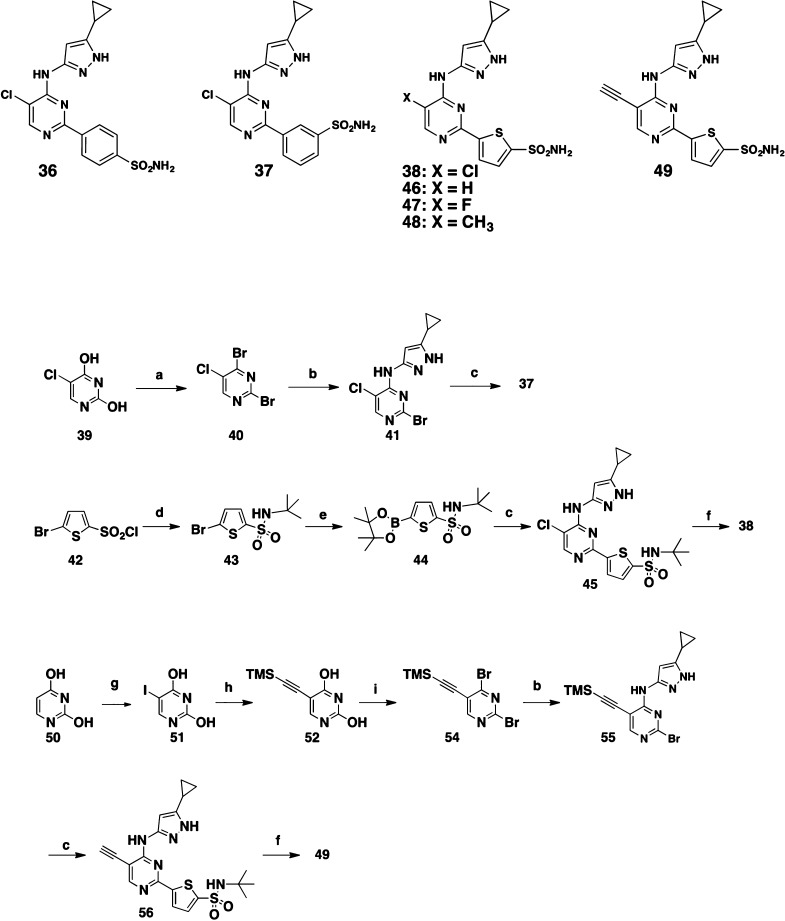
Scheme 2. Compounds 36–38 and 46–49 and the Representative Syntheses of 37, 38, and 49.
N,N-Dimethylaniline (2 equiv), POBr3 (3 equiv), toluene, 90 °C, 2 h, 83%.
5-Cyclopropyl-1H-pyrazol-3-amine, EtOH, RT, 4 h, 78%.
3- or 4-(Pinacolatoboranyl)-benzenesulfonamide for 36 and 37, or 44 for 38 and 56, Pd(dppf)Cl2 (0.1 equiv), Na2CO3 (4 equiv), dioxane/H2O (4:1), 100 °C, 15 h.
t-Butylamine, dioxane, 20 °C, 4 h, 94%.
Bis-pinacolatodiboron (1.2 equiv), Pd(dppf)Cl2 (0.1 equiv), CH3COOK (4.0 equiv), dioxane, 100 °C, 3 h, 70%.
BCl3, DCM, 20 °C, 6 h.
NIS, AcOH, RT, 7 h, 95%.
TMS-acetylene (1.2 equiv), (Ph3P)2PdCl2 (0.25 equiv), CuI (0.25 equiv), EtOAc, RT, 4 h, 46%.
N,N-Dimethylaniline, POBr3, 100 °C, 50 min, toluene, 51%.