The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG OUTCOME) trial demonstrated that one of sodium-glucose cotransporter 2 inhibitors (SGLT2i), empagliflozin, reduced all-cause death, death from cardiovascular causes and hospitalization for heart failure when given in addition to standard care in patients with type 2 diabetes at high cardiovascular risk (1), giving us a big surprise and a question to solve whether this result is a drug effect or a class effect.
Monami et al. reported a comprehensive meta-analysis of randomized placebo-controlled trials (RCTs) (2). Seventy-one trials were included (SGLT2i group, n=31,199; comparator group, n=16,088). SGLT2i was significantly related with a reduced incidence of all-cause mortality, cardiovascular mortality, and myocardial infarction, but not stroke, with no apparent difference across SGLT2i. However, the other meta-analysis including 81 RCTs (n=37,195) showed that the benefit was only obtained with empagliflozin (3). Further, the other meta-analysis (n=29,859) that compared 3 SGLT2i (canagliflozin, dapagliflozin and empagliflozin) to comparator groups also showed only empagliflozin was significantly associated with lower risk of major adverse cardiovascular events than comparator groups (4). However, such a significant effect of empagliflozin was greatly driven by the EMPA-REG OUTCOME trial. Other SGLT2i were not significantly associated with cardiovascular outcomes. Even meta-analyses could not answer whether cardioprotective effects of SGLT2i is due to a drug effect or a class effect. Further rigorous RCTs using SGLT2i other than empagliflozin had been awaited.
Recently, the Canagliflozin Cardiovascular Assessment Study (CANVAS) program reported the effects of canagliflozin on cardiovascular and renal events (5). The rate of a composite of death due to cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke was significantly lower with canagliflozin than with placebo (Table 1). Canagliflozin significantly reduced hospitalization for heart failure as compared with placebo by 33% as well as empagliflozin, supporting that cardioprotective effects of SGLT2i may be due to a class effect.
Table 1. Backgrounds and outcomes in EMPA-REG OUTCOME trial, CANVAS program and CVD-REAL study.
| Design, backgrounds and outcomes | Study | ||
|---|---|---|---|
| EMPA-REG OUTCOME trial | CANVAS program | CVD-REAL study | |
| Study design | RCT | Integrated data from 2 RCTs | Propensity-matched cohort study |
| N | 7,020 | 10,142 | 309,056 |
| Mean age (years) | 63 | 63 | 57 |
| Gender (male, approximately %) | 71 | 64 | 56 |
| A history of cardiovascular diseases (%) | >99 | 65.6 | 13 |
| Use of SGLT2 inhibitors (%) | Empagliflozin 100 | Canagliflozin 100 | Canagliflozin 53 |
| Dapagliflozin 42 | |||
| Empagliflozin 5 | |||
| Duration of follow-up | 3.1 years | 188.2 weeks | All-cause death |
| SGLT2i: 271 days | |||
| oGLD: 251 days | |||
| Hospitalization for heart failure | |||
| SGLT2i: 239 days | |||
| oGLD: 211 days | |||
| All-cause death (RR, %) | 32 | 13 (NS) | 51 |
| Death from cardiovascular causes (RR, %) | 14 | 14 | – |
| Hospitalization for heart failure (RR, %) | 35 | 33 | 39 |
| Hospitalization for heart failure or death from cardiovascular causes (RR, %) | 34 | 22 | – |
| Hospitalization for heart failure or all-cause death (RR, %) | – | – | 46 |
CANVAS, Canagliflozin Cardiovascular Assessment Study; CVD-REAL, Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors; EMPA-REG OUTCOME, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose; NS, not statistically significant; oGLD, other glucose-lowering drugs; RCT, randomized controlled trial; RR, risk reduction; SGLT2i, sodium-glucose cotransporter-2 inhibitors.
However, we cannot believe that the CANVAS program fully answered the question of whether the cardioprotective effect of SGLT2i is due to a drug effect or a class effect. Both EMPA-REG OUTCOME trial and CANVAS program showed that SGLT2i significantly reduced death from cardiovascular causes and hospitalization for heart failure. However, almost all of participants in EMPA-REG OUTCOME trial had established atherosclerotic cardiovascular diseases (CVD) and 66% of participants in CANVAS program had a history of CVD (Table 1). It remained unknown whether SGLT2i may reduce death and hospitalization for heart failure in type 2 diabetic patients without established CVD. The EMPA-REG OUTCOME trial presented a decrease of cardiovascular death and hospitalization for heart failure due to the treatment with empagliflozin within a short follow-up period. It remained unknown whether such an early separation of the event curves between empagliflozin- and placebo-treated patients can be observed in other SGLT2i.
The Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors (CVD-REAL) study answered the above three questions (6). This study was the first large real-world study of 309,056 type 2 diabetic patients which is approximately ten times larger than the number of eligible subjects in meta-analyses (2-4), and included patients with (13%) and without established CVD (87%) (Table 1). Further, the CVD-REAL study included patients treated with various kinds of SGLT2i such as canagliflozin (53%), dapagliflozin (42%), and empagliflozin (5%). The follow-up of the CVD-REAL study has relatively short duration which is similar to the period that early separation of the event curves was observed between empagliflozin- and placebo-treated patients. In the CVD-REAL study, the SGLT2i treatment was significantly associated with a risk reduction in hospitalization for heart failure, all-cause death, and hospitalization for heart failure or death composite as compared with other anti-diabetic drugs. I greatly agree with the CVD-REAL researchers’ conclusion that the benefits seen with empagliflozin in the EMPA-REG OUTCOME trial may be a class effect.
Although SGLT2i may improve metabolic risk factors which induce atherosclerosis (7), it is unlikely that the reduction of CV events can be explained by the improvement in metabolic risk factors. Hemodynamic factors may be more likely to reduce CV events. In type 2 diabetes, accumulated adipose tissue and resulting increase of insulin resistance increase angiotensin II which induces sodium retention (8). Insulin resistance also induces oxidative stress which increases salt sensitivity and sodium retention, leading to development of hypertension (8). Such an increased intravascular fluid volume and complication with hypertension are likely to develop heart failure in patients with type 2 diabetes.
The change in estimated glomerular filtration rate (eGFR) and the possible mechanisms for improvement in heart failure due to SGLT2i was shown in Figure 1 (9). In a very early phase (within 1–2 months) after the use of SGLT2i, eGFR remarkably decreased, in this phase osmotic diuresis reduces intravascular fluid volume, contributing to prevention of heart failure. Reduced blood pressure may continue to be associated with prevention of CVD.
Figure 1.
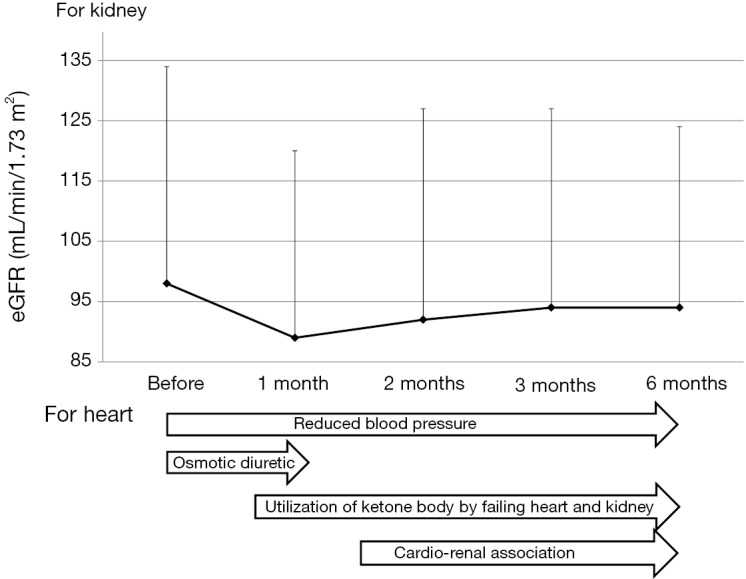
The change in estimated glomerular filtration rate (eGFR) and the possible mechanisms for improvement in heart failure due to sodium-glucose cotransporter 2 inhibitors (SGLT2i). The graph was modified from Figure 1 in (9).
SGLT2i may lead to glucose deficiency in patients with type 2 diabetes, and may induce elevation of fatty acid (FA) oxidation which increases the production of ketone bodies in liver (10). SGLT2i promote glucagon secretion which may be also associated with elevation of hepatic ketone bodies production (11). I believe that an increased formation of ketone bodies by SGLT2i may be associated with prevention of heart failure (12). Ferrannini et al. hypothesized that under a persistent hyperketonemia by SGLT2i, the heart uses ketone bodies rather than FA, and this fuel shift ameliorates cardiac work efficiency at the mitochondrial level as a “Thrifty Substrate Hypothesis” (13). Mudaliar et al. proposed a “Unifying Hypothesis” as cardio-renal protective effects of SGLT2i (14). Briefly, hearts and kidneys of diabetic patients cannot efficiently use FA or glucose as fuel, but can use an energy-efficient super fuel such as ketone bodies, and the energy source shift from FA/glucose to ketone bodies may improve cardiac and renal function (14).
Both EMPA-REG OUTCOME trial and CANVAS program showed that SGLT2i significantly improve renal function, which may be due to reduced blood pressure and intra-glomerular pressure, utilization of ketone bodies, a decrease of proximal tubules overload, the improvement in tubulointerstitial hypoxia and an increase of erythropoietin (5,9,15,16). The improvement of renal function may also ameliorate cardiac function through cardio-renal association.
Diuretics such as loop diuretics are used to treat heart failure. However, observational studies have indicated that diuretics may be harmful in heart failure, and may be also associated with worsening renal function (17). SGLT2i may treat heart failure without worsening renal function, but with improving renal function. In healthy humans, ketone body (3-hydroxybutyrate) has been shown to decrease myocardial glucose uptake and to elevate myocardial blood flow, suggesting that ketone bodies are crucial fuels for heart and ketone bodies work as vasodilators. This proposes potentials of ketone bodies for the treatment of heart failure (18), supporting the usefulness of SGLT2i for treatment of heart failure.
In summary, the CVD-REAL study (6) showed a significant contribution of SGLT2i to a risk reduction in hospitalization for heart failure and all-cause death, and also proved that the benefits seen with empagliflozin in the EMPA-REG OUTCOME trial may be a class effect. The CVD-REAL study also gave us the chance to think about the underlying-mechanisms of development of heart failure in patients with type 2 diabetes. Reduced blood pressure may continuously prevent development of heart failure. In an early phase after the start of SGLT2i, osmotic diuresis may play a crucial role in preventing heart failure. After that, utilization of ketone bodies by failing heart/kidney may contribute to prevention of heart failure, and in the relatively late phase after the start of SGLT2i, a favorable cardio-renal association due to renoprotective effects of SGLT2i may be associated with prevention of heart failure. I believe that SGLT2i can contribute to effective and safe treatment for heart failure in a broad population of patients with type 2 diabetes.
Acknowledgements
None.
Provenance: This is an invited Editorial commissioned by Section Editor Dr. Kaiping Zhang, PhD (AME College, AME Group, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117-28. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 2.Monami M, Dicembrini I, Mannucci E. Effects of SGLT-2 inhibitors on mortality and cardiovascular events: a comprehensive meta-analysis of randomized controlled trials. Acta Diabetol 2017;54:19-36. 10.1007/s00592-016-0892-7 [DOI] [PubMed] [Google Scholar]
- 3.Saad M, Mahmoud AN, Elgendy IY, et al. Cardiovascular outcomes with sodium-glucose cotransporter-2 inhibitors in patients with type II diabetes mellitus: a meta-analysis of placebo-controlled randomized trials. Int J Cardiol 2017;228:352-8. 10.1016/j.ijcard.2016.11.181 [DOI] [PubMed] [Google Scholar]
- 4.Tang H, Fang Z, Wang T, et al. Meta-analysis of effects of sodium-glucose cotransporter 2 inhibitors on cardiovascular outcomes and all-cause mortality among patients with type 2 diabetes mellitus. Am J Cardiol 2016;118:1774-80. 10.1016/j.amjcard.2016.08.061 [DOI] [PubMed] [Google Scholar]
- 5.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644-57. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 6.Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation 2017;136:249-59. 10.1161/CIRCULATIONAHA.117.029190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanai H, Katsuyama H, Hamasaki H, et al. Sodium-glucose cotransporter 2 inhibitors: possible anti-atherosclerotic effects beyond glucose lowering. J Clin Med Res 2016;8:10-4. 10.14740/jocmr2385w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanai H, Tomono Y, Ito K, et al. The underlying mechanisms for development of hypertension in the metabolic syndrome. Nutr J 2008;7:10. 10.1186/1475-2891-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanai H, Katsuyama H. A possible mechanism for renoprotective effect of sodium-glucose cotransporter 2 inhibitor: elevation of erythropoietin production. J Clin Med Res 2017;9:178-9. 10.14740/jocmr2857w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashiwagi A, Maegawa H. Metabolic and hemodynamic effects of sodium-dependent glucose cotransporter 2 inhibitors on cardio-renal protection in the treatment of patients with type 2 diabetes mellitus. J Diabetes Investig 2017;8:416-27. 10.1111/jdi.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015;100:2849-52. 10.1210/jc.2015-1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanai H. Sodium-glucose cotransporter 2 inhibitors for heart failure. J Clin Hypertens (Greenwich) 2017;7:75-6. 10.14740/jem426w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a "Thrifty Substrate" Hypothesis. Diabetes Care 2016;39:1108-14. 10.2337/dc16-0330 [DOI] [PubMed] [Google Scholar]
- 14.Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care 2016;39:1115-22. 10.2337/dc16-0542 [DOI] [PubMed] [Google Scholar]
- 15.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323-34. 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 16.Sano M, Takei M, Shiraishi Y, et al. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res 2016;8:844-7. 10.14740/jocmr2760w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael Felker G. Diuretic management in heart failure. Congest Heart Fail 2010;16 Suppl 1:S68-72. 10.1111/j.1751-7133.2010.00172.x [DOI] [PubMed] [Google Scholar]
- 18.Gormsen LC, Svart M, Thomsen HH, et al. Ketone body infusion with 3-hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: a positron emission tomography study. J Am Heart Assoc 2017;6(3). 10.1161/JAHA.116.005066 [DOI] [PMC free article] [PubMed] [Google Scholar]


