Figure 6.
Unfolded Proteins Compete for BiP to Restore the IRE1 Dimer
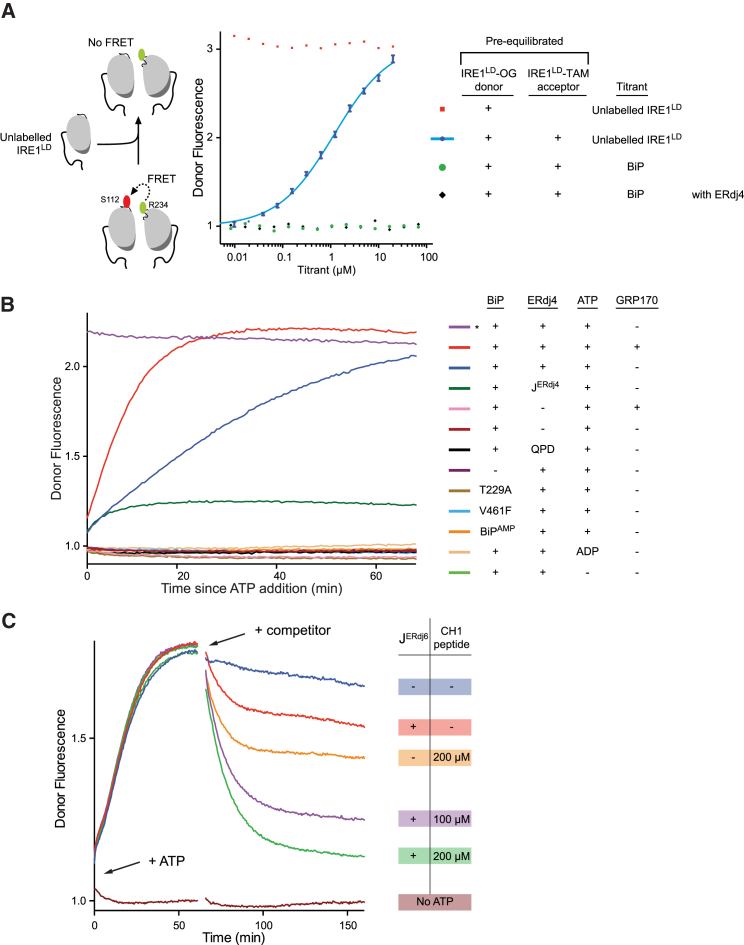
(A) Donor fluorescence as a function of the concentration of competing unlabeled IRE1LD equilibrated with a FRET pair (0.2 μM labeled IRE1LD) consisting of an IRE1LD-OG488 donor (conjugated at R234C) and IRE1LD-TAM acceptor (conjugated at S112C) (blue trace, mean values ± SD from three independent experiments). Also shown are titrations of unlabeled IRE1LD into a mock FRET sensor (no IRE1LD-TAM acceptor; red trace) and titration of BiP with ADP (± ERdj4) into the pre-equilibrated FRET pair (green and black traces).
(B) Time-dependent change in donor fluorescence of the IRE1LD FRET pair from (A) incubated at t = 0 with the components shown to the right. Concentrations used were 0.2 μM FRET IRE1LD, 30 μM BIP, 2.5 μM ERdj4, 1 μM GRP170, and 2 mM ATP. JERdj4 lacks the C-terminal targeting region. BiPAMP is AMPylated BiP. The asterisks marks a reaction set up with a mock FRET sensor lacking the IRE1LD-TAM acceptor.
(C) Time-dependent change in donor fluorescence of the IRE1LD FRET pair exposed at t = 0 to BiP, ERdj4 and ATP (arrow labeled “+ ATP”). Concentrations used were 0.2 μM FRET IRE1LD, 50 μM BIP, 2.5 μM ERdj4, and 2 mM ATP. Following disruption of the FRET pair, at 60 min, the sample was injected with BiP binding peptide and the J domain of ERdj6 (2.5 μM) (arrow labeled “+ competitor”).
See also Figure S5.