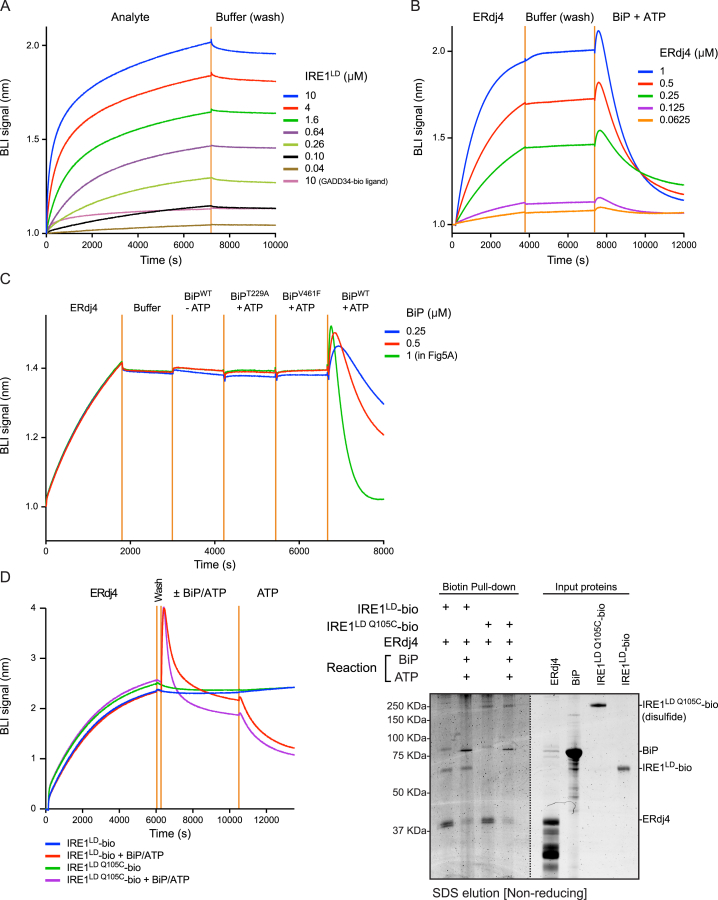
Figure S4.
Disruption of Pre-formed IRE1LD-Dimers, Related to Figure 5
(A) Bio-layer interferometry (BLI) signal from streptavidin sensors pre-loaded with a biotinylated ERdj4 ligand (or with an irrelevant control biotinylated GADD34 ligand) and reacted with the indicated concentration of IRE1LD as an analyte and then transferred to a buffer only (wash) solution.
(B) BLI signal from streptavidin sensors pre-loaded with biotinylated IRE1LD ligand and reacted with the indicated concentrations of ERdj4 as an analyte and transferred to a buffer only (wash) solution before incubation with 1 μM BiP and 2 mM ATP.
(C) BLI signal from streptavidin sensors pre-loaded with biotinylated IRE1LD ligand and reacted with ERdj4 as an analyte and then transferred to a buffer only (wash) solution before incubation with the indicated concentrations of BiP and 2 mM ATP.
(D) (Left) BLI signal of streptavidin sensors loaded with the wild-type biotinylated IRE1LD, or covalent dimeric disulfide-linked biotinylated IRE1LD Q105C ligands and reacted with ERdj4, followed sequentially by the indicated solutions. Concentrations used were 1.7 μM ERdj4, 6 μM BiP, 2 mM ATP. (Right) Coomasie-stained non-reducing SDS-PAGE gel of protein recovered by SDS sample buffer elution from the BLI sensors used (left). The dotted line indicates the boundary at which the image contrast/brightness properties were treated differently to make the image clearer. Note: To enable formation of Q105C-disulfide, without interference by other cysteines, both the WT IRE1LD and the IRE1LD Q105C ligands were surface biotinylated on exposed lysine residues. This coupling chemistry likely accounts for the differences in kinetics of the BLI signal observed in this experiment as compared with (A), (B), and (C) and Figure 5A, in which the IRE1LD ligand was biotinylated on a single C-terminal cysteine residue (D443C) using maleimide biotin