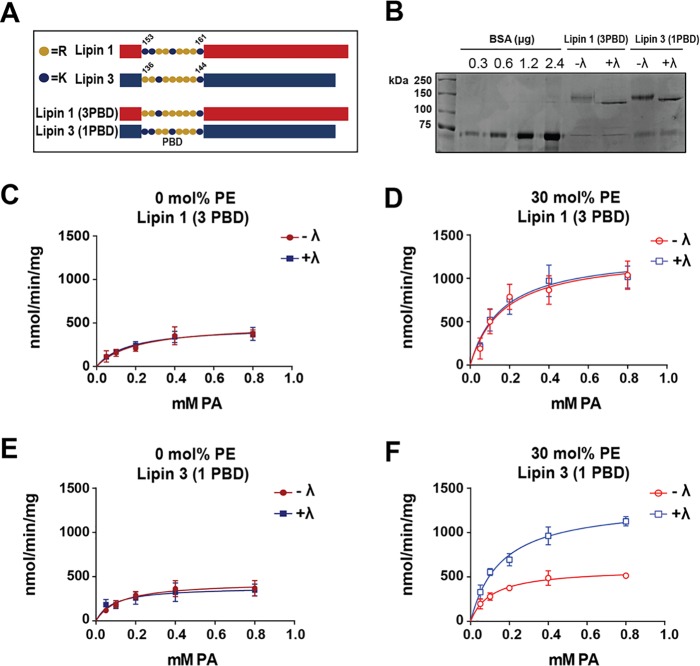
Figure 4.
Enzymatic activity of lipin PBD exchange mutants in liposomes. A, schematic of lipin PBD exchange mutants. B, PBD mutants were expressed, affinity-purified, incubated in phosphatase buffer with (+λ) or without (−λ) λ phosphatase, and eluted as described before. Proteins were separated on an SDS-polyacrylamide gel along with BSA standards and stained with Coomassie Blue dye. The PAP activities of purified phosphorylated (−λ) and dephosphorylated (+λ) lipin 1 (3PBD) were measured with PC/PA (C) and PC/PE/PA (D) liposomes. Shown are the PAP activities of −λ and +λ purified lipin 3 (1PBD) with PC/PA (E) and PC/PE/PA (F) liposome. Activities were assayed at pH 7.5. Each data point is a mean of triplicate determinations ± S.E. (error bars).