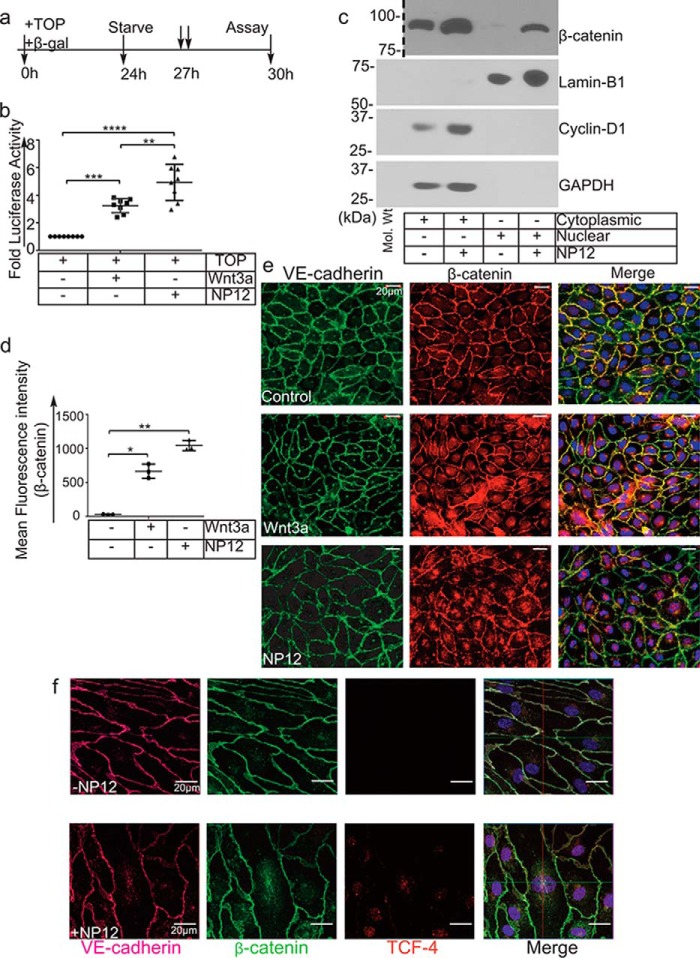
Figure 4.
Allosteric inhibition of GSK-3β mimics canonical Wnt signaling. a, timeline of the TOPFlash luciferase assay. b, ECs were transiently transfected with the TOPFlash plasmid and reporter β-gal and thereafter treated with (↓↓) Wnt3a or NP12 for 3 h. The TCF4-promoter luciferase activity following NP12 treatment is presented as fold luciferase activity; experiments were performed four times with two technical replicates, and each data point represents one technical sample; thereafter, data were subjected to ANOVA followed by Sidak's test. c, cytoplasmic and nuclear fractions prepared from control ECs or ECs receiving NP12 (60 nmol/liter for 6 h) were analyzed by immunoblotting with β-catenin and cyclin-D1 antibodies. Dotted line indicates the position where the nitrocellulose membrane was cut. The cytoplasmic and nuclear fractions prepared from NP12-treated ECs showed increased accumulation of β-catenin in both compartments, whereas cyclin-D1 was primarily detected in the cytoplasmic fraction. For loading controls, we used GAPDH and lamin-B1. d and e, control ECs (vehicle) or ECs receiving NP12 (60 nmol/liter) were fixed and stained with anti-VE-cadherin (green) and anti-β-catenin (red). Control ECs showed β-catenin (red) largely at the membrane; in contrast, NP12-treated ECs showed increased cytoplasmic, some perinuclear, and nuclear staining (×40). Each data point represents the mean value, and each experiment was performed with three technical replicates; thereafter, data were subjected to ANOVA followed by Dunnett's test. f, high-resolution (×63) confocal z-stack imaging showed co-localization of β-catenin (green) with TCF4 (red) in the nucleus of NP12-treated ECs as compared with that observed in controls. VE-cadherin (magenta). Error bars represent ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001 versus indicated groups. Scale bars are as shown.