Abstract
Introduction
Posttransplantation diabetes mellitus (PTDM) is a common complication among kidney transplant recipients and is associated with a higher risk of cardiovascular events and poorer graft and patient survival. The association of pretransplantation hemoglobin A1c (HbA1c) with PTDM remains unclear. Identifying recipients at greatest risk for PTDM may help guide monitoring and treatment strategies to prevent or delay the onset of PTDM.
Methods
We analyzed data from 1499 nondiabetic primary kidney transplant recipients with available pretransplantation HbA1c values in the United States Renal Data System (USRDS) from 2005 to 2011. Recipients with pretransplantation diabetes diagnosis or HbA1c ≥ 6.5% were excluded. We assessed the association of pretransplantation HbA1c with PTDM using Cox proportional hazards models. Pretransplantation HbA1c level as a continuous variable was modeled using restricted cubic splines with knots at the 25th, 50th, and 75th percentiles. Based on results from this model, pretransplantation HbA1c was further modeled using a linear spline with a single knot at 5.4%.
Results
A total of 395 recipients (26.4%) developed PTDM over a median follow-up of 1.8 years. Pretransplantation HbA1c was not significantly associated with risk of PTDM below 5.4%, whereas each 1% higher HbA1c above 5.4% was associated with an adjusted hazard ratio of 1.84 (95% confidence interval = 1.28, 2.66; P for change in slope = 0.04).
Discussion
Higher pretransplantation HbA1c above 5.4% is independently associated with greater risk of PTDM among kidney transplant recipients. A continuous relationship between pretransplantation HbA1c and risk of PTDM suggests that increased risk starts at HbA1c levels well below current thresholds for prediabetes.
Keywords: clinical epidemiology, diabetes mellitus, HbA1c, kidney transplantation
Posttransplantation diabetes mellitus (PTDM) is a common metabolic complication after kidney transplantation. Its incidence varies, depending on the characteristics of the relevant population, the diagnostic criteria used for PTDM, and immunosuppressive regimen. The reported cumulative incidence of PTDM is approximately 10% to 20% at 1 year and 25% to 35% at 3 years after kidney transplantation.1, 2 Development of PTDM is associated with unfavorable clinical outcomes, including higher risk of cardiovascular events, inferior patient and graft survival, and poorer quality of life.3, 4, 5 Identification of patients who are at high risk for PTDM before transplantation may help guide monitoring and treatment strategies to prevent or delay the incidence of PTDM.
Pretransplantation insulin resistance significantly increases the risk of PTDM.6 Impaired fasting glucose and impaired glucose tolerance before transplantation are both associated with greater risk of PTDM.7, 8 Therefore, the consensus guideline by the International Expert Panel recommends that all kidney transplant candidates have an annual check of glycemic status, either in the form of fasting glucose or a risk-stratified oral glucose tolerance test based on the center-specific screening algorithm.9 However, oral glucose tolerance testing is not widely used, as it is time consuming and impractical in large transplantation programs.
Measuring hemoglobin A1c (HbA1c) in blood is much more convenient than an oral glucose tolerance test, and is widely accepted in clinical practice as the recommended means of diagnosing diabetes and identifying individuals in the general population that are at high risk of diabetes (i.e., prediabetes).10, 11 Recently, the HbA1c test was added as 1 of the recommended methods to diagnose PTDM by the International Expert Panel at the Consensus Meeting on PTDM in 2013.9 HbA1c levels are less reliable in patients with impaired kidney function than in the general population due to elevated blood urea levels, anemia, shortened erythrocyte survival time, or erythropoietin treatment.12 Thus, the relationship between pretransplantation HbA1c level and risk of PTDM among kidney transplant recipients is unclear.
To address this question, we examined the association of pretransplantation HbA1c with PTDM over a follow-up period of up to 5 years in 1499 primary nondiabetic kidney transplant recipients.
Materials and Methods
Data Sources
The United States Renal Data System (USRDS), a national data system that collects information on end-stage renal disease (ESRD) in the United States, was used for this study. Information was also obtained from the transplant database from the United Network for Organ Sharing, Medicare claims data, and the revised Center for Medicaid and Medicare Services (CMS) Form 2728 (ESRD Medical Evidence Report) introduced in May 2005, which started collecting data on blood HbA1c and lipid profiles before the most recent ESRD episode.
Study Population
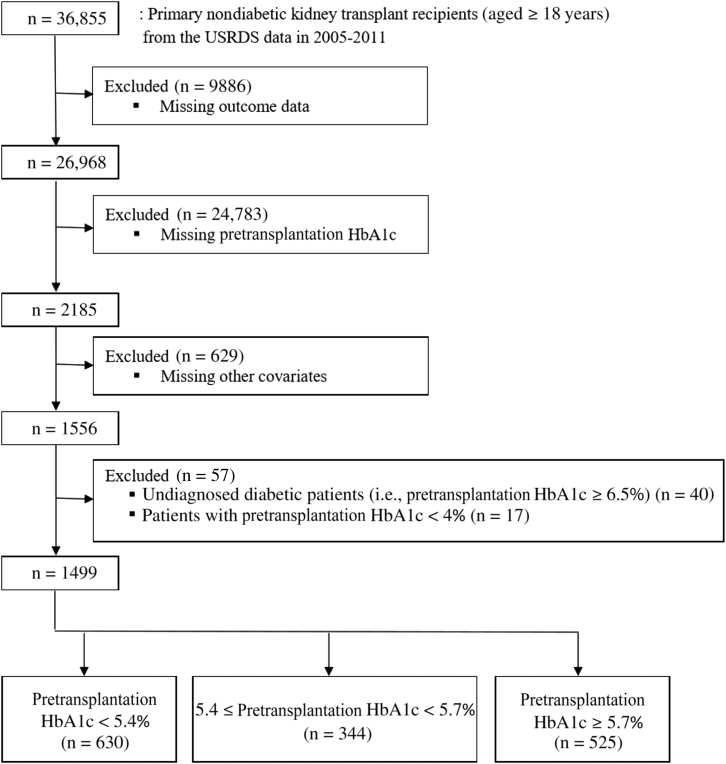
All nondiabetic adults who received a primary kidney transplant between 1 May 2005 and 31 December 2011 were eligible for inclusion in this study. Recipients with diabetes based on information from the CMS Form 2728 (question 17: co-morbid conditions [i.e., diabetes, currently on insulin; diabetes, currently on oral medications; diabetes, without medications; diabetic retinopathy] or question 15: primary cause of renal failure), the United Network for Organ Sharing transplant candidate registration form (general medical factors: diabetes), or USRDS Medicare claims data (International Classification of Diseases, Ninth Edition, Clinical Modification [ICD-9-CM] diabetes diagnosis codes) were excluded. Of 36,855 recipients not diagnosed with diabetes, 9886 (26.8%) were excluded due to missing data on outcomes (i.e., unavailable Medicare claims data after transplantation) (Figure 1). Of 26,968 nondiabetic recipients with obtainable outcome data, pretransplantation HbA1c was available for 2185 (8.1%). Of these individuals, 629 were excluded due to missing data on other covariates. Of the remaining 1556 recipients, 40 had undiagnosed diabetes (pretransplantation HbA1c ≥ 6.5%) and were excluded. An additional 57 recipients had HbA1c ≤ 4% and were excluded. The remaining 1499 recipients were followed up until the development of PTDM, graft loss (i.e., death or graft failure before death), or 31 March 2012. This study was approved by the University of Wisconsin Health Sciences Institutional Review Board.
Figure 1.
Flow diagram of the sample in the study.
Exposure and Outcome Variables
Blood HbA1c level measured before the date of kidney transplantation was ascertained from the laboratory values on CMS form 2728. PTDM was ascertained by ICD-9-CM codes in the USRDS Medicare claims data and a previously validated method.13 An ICD-9-CM diagnosis code 249 (i.e., secondary diabetes mellitus) or 250 (i.e., diabetes mellitus) reported after the date of kidney transplantation was used to detect the development of PTDM. The date of onset of diabetes was assumed to be the date of the earliest claim.
Definition of Variables
Prediabetes was defined as pretransplantation HbA1c ≥ 5.7%.10, 11 The definition of the metabolic syndrome by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III)14 was slightly modified because of the lack of variables (i.e., waist circumference, fasting plasma glucose, and blood pressure). For our study, it was defined as having at least 3 of the following 5 conditions: generalized obesity (body mass index [BMI] ≥ 30 kg/m2) as a surrogate marker for central obesity,15 history of hypertension for blood pressure ≥ 130/85 mm Hg, elevated HbA1c (HbA1c ≥ 5.7%) for fasting plasma glucose ≥ 110 mg/dl,11, 16 high triglyceride (TG) level (TG ≥ 150 mg/dl), and low high-density lipoprotein (HDL) cholesterol level (<40 mg/dl in men and <50 mg/dl in women).
Statistical Analysis
Participants were divided into 3 groups using the 50th percentile of pretransplantation HbA1c (5.4%) and the current threshold of HbA1c for prediabetes (5.7%). Baseline characteristics of patients were compared to assess a trend across the 3 ordered groups (i.e., HbA1c < 5.4%; 5.4 ≤ HbA1c < 5.7%; HbA1c ≥ 5.7%) using the nonparametric test, an extension of the Wilcoxon rank-sum test for continuous variables, and the Cochran−Armitage test for categorical variables.
Participants were censored at death, graft failure before death, or end of study (31 March 2012). Incidence of PTDM was assessed with Kaplan−Meier analysis and compared among the 3 groups using log-rank tests. The association of pretransplantation HbA1c level as a continuous variable with PTDM was modeled using restricted cubic splines with knots at the 25th, 50th, and 75th percentiles (5.1%, 5.4%, and 5.6%, respectively). Based on results from this model, pretransplantation HbA1c level was further modeled by a multivariate linear spline Cox model with a single knot at 5.4%. The goodness of fit of 2 models (i.e., a cubic spline model with 3 knots vs. a linear spline model with a single knot) were compared using a likelihood ratio test. Based on the continuous relationship between HbA1c and PTDM, HbA1c level was dichotomized (< 5.4 vs. ≥ 5.4%). The unadjusted and adjusted associations of HbA1c ≥ 5.4% with PTDM were assessed with Cox proportional hazards regression models. All the Cox models accounted for potential center-level clustering.
Confounding variables in our multivariate regression models comprised factors potentially associated with PTDM and/or HbA1c, including demographics, pretransplantation medical conditions, and transplant-specific factors based on the prior knowledge.17 The following potential confounding variables were included in the final model: (i) demographics: age in years (continuous), sex, race/ethnicity (non-Hispanic white; Hispanic; black; other); (ii) pretransplantation medical conditions: cause of ESRD (glomerulonephritis; polycystic kidney disease; hypertension; other), history of cardiovascular disease and hypertension (yes/no for each), duration of pretransplantation dialysis (continuous), body mass index (BMI; continuous), serum triglyceride (TG; continuous) and high-density lipoprotein (HDL; continuous), blood hemoglobin level (continuous), erythropoietin use (yes/no); and (iii) transplant-specific factors: year of transplantation (continuous), number of prior transplants (0 or ≥ 1), number of human leukocyte antigen (HLA) mismatch (continuous), preemptive transplant (yes/no) donor type (living/ deceased), donor age in years (continuous), hepatitis C virus (HCV) and cytomegalovirus (CMV) serostatus (positive/negative for each), immunosuppressive induction agents (antithymocyte globulin, alemtuzumab, interleukin-2 receptor antagonist, steroid: yes/no for each), posttransplantation maintenance immunosuppressive agent (steroid, tacrolimus, cyclosporin, mycophenolate mofetil, mammalian target of rapamycin (mTOR) inhibitors: yes/no for each). In addition to these a priori−selected variables, time between HbA1c measurement and date of transplantation in months (continuous) was included after exploratory data analyses.
Analyses were repeated with stratification by (i) an a priori set of variables including erythropoietin use, presence of anemia (i.e., blood hemoglobin level< 11 mg/dl), immunosuppressive induction agents, steroid maintenance therapy, donor type, presence of the metabolic syndrome and its individual components (i.e., obesity, history of hypertension, low HDL cholesterol, elevated TG level); and (ii) additional variables selected based on findings from the comparison between included recipients and those excluded from the study: age (≥ 50 [median] or < 50 years) and race (black/nonblack) categories. Statistical significance of interaction between HbA1c and each interacting variable was assessed.
Sensitivity Analyses
Two separate analyses were conducted to evaluate whether pretransplantation HbA1c was associated with early onset of PTDM (i.e., within 1 year after transplantation) as well as later onset of PTDM (i.e., beyond 1 year after transplantation). The first analysis was limited to 1-year follow-up after transplantation (i.e., the recipients without development of PTDM were censored at the end of the first year). The second analysis was carried out only for recipients free of PTDM at the end of the first year, with follow-up starting 1 year after transplantation to the end of study.
Similarly, analyses were repeated starting follow-up at 45 days after transplantation to exclude possible transient posttransplantation hyperglycemia, as the International Expert Panel recommended not to make a diagnosis of PTDM within 45 days after transplantation.9 Additional analyses were performed limited to those recipients who had HbA1c level measured within 1 year before transplantation.
Proportionality assumptions in the Cox model were checked graphically based on Schoenfeld residuals, and those assumptions were met in all the covariates in the model. The results of survival analyses are shown as hazard ratio (HR) with 95% confidence interval (CI). All analyses were conducted using Stata/MP 13.1 (www.stata.com).
Results
Recipients excluded from the analyses were slightly younger (47.2 vs. 48.6 years; P < 0.001), were more likely to be black (17.8 vs. 13.9%; P < 0.001), and had slightly higher HDL cholesterol level (42.8 vs. 41.3 mg/dl; P < 0.001) than their counterparts included in the analyses (Table 1). Sex, year of transplantation, BMI, prevalence of obesity and morbid obesity (BMI ≥ 40 kg/m2), serum TG level, history of hypertension, and history of cardiovascular disease were comparable between the included and excluded recipients. The incidence of PTDM was also comparable between the included and excluded recipients. The results from the comparison between recipients with HbA1c and without HbA1c before transplantation were similar (Supplementary Table S1). Preemptive transplantation was the only independent predictor of reporting HbA1c despite not being diabetic, with an odds ratio of 1.91 (95% CI = 1.35, 2.68), in the logistic regression model adjusting for age, sex, race/ethnicity, year of transplantation, BMI, serum TG and HDL-cholesterol level, history of hypertension and cardiovascular disease, and center-level clustering.
Table 1.
Comparison of characteristics and incidence of PTDM between recipients included and those excluded from the study
| Included recipients (n = 1499) |
Excluded recipients (n = 35,356) |
P value | |
|---|---|---|---|
| Characteristic | |||
| Age, yr | 48.6 (14.4) | 47.2 (14.3) | <0.001 |
| Female, % | 40.7 | 41.6 | 0.50 |
| Race/ethnicity, % | |||
| Non-Hispanic white | 71.4 | 66.5 | <0.001 |
| Hispanic | 10.3 | 10.3 | |
| Black | 13.9 | 17.8 | |
| Other | 4.5 | 5.4 | |
| Year of transplantation, % | |||
| 2005–2008 | 47.0 | 46.2 | 0.58 |
| 2009–2011 | 53.0 | 53.8 | |
| BMI, kg/m2 | 27.4 (5.5) | 27.1 (5.4)a | 0.58 |
| Obesity (BMI ≥ 30 kg/m2), % | 28.9 | 28.3a | 0.61 |
| Morbid obesity (BMI ≥ 40 kg/m2), % | 2.3 | 1.7a | 0.11 |
| Serum triglyceride, mg/dl | 175.4 (131.2) | 173.8 (152.0)b | 0.70 |
| Serum HDL-cholesterol, mg/dl | 41.3 (15.3) | 42.8 (15.5)c | <0.001 |
| History of hypertension, % | 83.1 | 81.9 | 0.25 |
| History of cardiovascular disease, % | 12.5 | 13.3 | 0.38 |
| Outcome | |||
| N at risk | 1499 | 25,470d | − |
| Number of events | 395 | 6613 | |
| Incidence rate, per 100 PYs | 13.6 | 13.2 | 0.54 |
| Cumulative incidence, % | |||
| At 1 year | 17.4 | 17.3 | 0.82 |
| At 3 years | 30.5 | 29.8 | 0.69 |
| At 5 years | 40.8 | 40.7 | 0.77 |
Values are mean (SD) unless otherwise indicated.
BMI, body mass index; HDL, high-density lipoprotein; PTDM, posttransplantation diabetes mellitus; PYs, person-years.
n = 35,317.
n = 10,739.
n = 10,105.
Adding the number of excluded recipients (n = 25,470) to those included in the study (n = 1449) does not give 36,855, the total number of nondiabetic recipients, because unavailable outcome data was 1 of the exclusion criteria.
Of the 1499 recipients included in the analyses, 525 (35.0%) had prediabetes (HbA1c ≥ 5.7%) before kidney transplantation, and 344 (23%) had HbA1c values in the range 5.4 ≤ HbA1c < 5.7%. Mean values of pretransplantation HbA1c across the 3 groups (< 5.4 vs. 5.4 ≤ HbA1c < 5.7 vs. ≥ 5.7%) were 5.0, 5.5, and 6.0, respectively (P-trend < 0.001). The mean of HbA1c appeared to be slightly lower among erythropoietin users than among nonusers, but it was not statistically significant (5.40 vs. 5.45; P = 0.07). The overall median interval between the date of HbA1c measurement and the date of transplantation was 61 days (interquartile range = 434 days), and the median intervals across the 3 groups were 23, 73, and 104 days, respectively (P-trend < 0.001). Higher HbA1c was significantly associated with older age, higher proportion of male sex, higher prevalence of cardiovascular disease, longer duration of pretransplantation dialysis, more recent years of transplantation, and lower proportion of HCV positivity (all P-trend < 0.05) (Table 2).
Table 2.
Baseline characteristics of patients, by pretransplantation HbA1c level
| Total | HbA1c < 5.4% | 5.4 ≤ HbA1c < 5.7% | HbA1c ≥ 5.7% | P-trend | |
|---|---|---|---|---|---|
| n (%) | 1499 (100.0) | 630 (42.0) | 344 (23.0) | 525 (35.0) | |
| HbA1c (%) | 5.43 (0.49) | 4.97 (0.31) | 5.50 (0.08) | 5.95 (0.21) | <0.001 |
| Date of transplantation-date of HbA1c measured, days, median (interquartile range) | 61 (434) | 23 (382) | 73 (356) | 104 (542) | 0.001 |
| Age, yr | 48.6 (14.4) | 46.7 (14.6) | 48.6 (13.9) | 50.9 (14.1) | <0.001 |
| Female, % | 40.7 | 44.3 | 38.1 | 38.1 | 0.03 |
| Race/ethnicity, % | |||||
| Non-Hispanic white | 71.4 | 71.9 | 71.8 | 70.5 | 0.28 |
| Hispanic | 10.3 | 11.7 | 10.2 | 8.6 | 0.08 |
| Black | 13.9 | 13.5 | 12.8 | 15.0 | 0.46 |
| Other | 4.5 | 2.9 | 5.2 | 5.9 | 0.02 |
| History of cardiovascular disease, % | 12.5 | 10.6 | 9.6 | 16.6 | 0.03 |
| Cause of end-stage renal disease, % | |||||
| Glomerulonephritis | 24.1 | 23.0 | 25.6 | 24.4 | 0.57 |
| Polycystic kidney disease | 18.6 | 17.1 | 21.5 | 18.5 | 0.52 |
| Hypertension | 20.7 | 19.7 | 20.1 | 22.3 | 0.29 |
| Other | 36.6 | 40.2 | 32.8 | 34.9 | 0.06 |
| Preemptive transplantation, % | 58.0 | 57.0 | 60.2 | 57.7 | 0.77 |
| Duration of pretransplantation dialysis, yr, median (interquartile range) | 1.5 (1.9) | 1.3 (1.9) | 1.3 (2.0) | 1.6 (1.8) | 0.03 |
| Year of transplantation, % | |||||
| 2005–2008 | 47.0 | 48.7 | 51.2 | 42.1 | 0.03 |
| 2009–2011 | 53.0 | 51.3 | 48.8 | 57.9 | 0.03 |
| Retransplantation, % | 8.0 | 9.1 | 7.3 | 7.2 | 0.25 |
| HLA mismatch | 3.4 (1.8) | 3.5 (1.8) | 3.2 (1.9) | 3.4 (1.7) | 0.47 |
| Living donor, % | 63.2 | 61.4 | 68.0 | 62.3 | 0.70 |
| Donor age, yr | 40.6 (13.5) | 40.6 (13.5) | 41.0 (13.3) | 40.5 (13.8) | 0.77 |
| HCV positive serology, % | 3.2 | 4.4 | 2.3 | 2.3 | 0.03 |
| CMV positive serology, % | 53.6 | 52.9 | 50.9 | 56.4 | 0.25 |
| Induction agenta, % | |||||
| Antithymocyte globulin | 46.6 | 45.1 | 45.6 | 49.1 | 0.17 |
| Alemtuzumab | 8.9 | 9.8 | 9.3 | 7.4 | 0.16 |
| Interleukin-2 receptor antagonist | 34.0 | 35.1 | 34.6 | 32.4 | 0.34 |
| Steroid | 72.5 | 71.8 | 73.6 | 72.8 | 0.68 |
| Maintenance immunosuppressive agenta, % | |||||
| Steroid | 60.3 | 60.2 | 60.8 | 60.2 | 0.98 |
| Tacrolimus | 87.1 | 85.7 | 88.4 | 87.8 | 0.28 |
| Cyclosporin | 8.8 | 9.1 | 10.2 | 7.6 | 0.42 |
| Mycophenolate mofetil | 93.1 | 93.2 | 91.0 | 94.5 | 0.43 |
| mTOR inhibitor | 6.6 | 6.2 | 7.3 | 6.7 | 0.73 |
| Blood hemoglobin, mg/dl | 10.7 (1.8) | 10.7 (1.8) | 10.8 (1.8) | 10.8 (1.9) | 0.10 |
| Erythropoietin use, % | |||||
| Yes | 35.5 | 38.6 | 36.0 | 31.4 | 0.01 |
| No | 64.5 | 61.4 | 64.0 | 68.6 | 0.01 |
Values are mean (SD) unless otherwise indicated.
CMV, cytomegalovirus; HCV, hepatitis C virus; HLA, human leukocyte antigen; mTOR, mammalian target of rapamycin.
Not exclusive categories.
Higher HbA1c was significantly associated with higher prevalence of the metabolic syndrome (36.7% vs. 40.7% vs. 75.8%; P-trend < 0.001) (Table 3). Among the 4 components of the metabolic syndrome other than HbA1c, BMI (26.9 vs. 27.8 vs. 27.7 kg/m2; P-trend = 0.01) and serum TG level (162.5 vs. 173.8 vs. 192.0 mg/dl; P-trend < 0.001) were significantly associated with higher HbA1c.
Table 3.
Components of metabolic syndrome among patients, by pretransplantation HbA1c level
| Variable | Total | HbA1c < 5.4% | 5.4 ≤ HbA1c < 5.7% | HbA1c ≥ 5.7% | P-trend |
|---|---|---|---|---|---|
| Body mass index, kg/m2 | 27.4 (5.5) | 26.9 (5.3) | 27.8 (5.4) | 27.7 (5.6) | 0.01 |
| Serum triglyceride, mg/dl | 175.4 (131.2) | 162.5 (106.8) | 173.8 (114.9) | 192.0 (162.5) | <0.001 |
| Serum HDL-cholesterol, mg/dl | 41.3 (15.3) | 41.1 (16.3) | 41.1 (16.4) | 41.7 (14.7) | 0.45 |
| Metabolic syndrome, % | 51.3 | 36.7 | 40.7 | 75.8 | <0.001 |
| Body mass index ≥ 30 kg/m2, % | 29.0 | 26.5 | 31.7 | 30.1 | 0.16 |
| History of hypertension, % | 83.1 | 82.5 | 83.4 | 83.4 | 0.68 |
| Serum triglyceride ≥ 150 mg/dl, % | 46.6 | 43.5 | 45.4 | 51.2 | 0.01 |
| Low HDL-cholesterola, % | 63.2 | 63.0 | 65.4 | 61.9 | 0.73 |
Values are mean (SD) unless otherwise indicated.
HDL, high-density lipoprotein.
Low HDL-cholesterol was defined as < 40 mg/dl in men and < 50 mg/dl in women.
Pretransplantation HbA1c Level and Risk of PTDM
A total of 395 patients (26.4%) developed PTDM over a median follow-up of 1.8 years (incidence rate =13.59 events/100 person-years). Of 395 events, 247 (62.5%) occurred during the first year after transplantation (incidence rate = 20.50 events/100 person-years), and 148 (37.5%) occurred beyond the first year after transplantation (incidence rate = 5.40 events/100 person-years). A total of 39 events (9.9%) occurred within 45 days after transplantation. The cumulative incidences of PTDM were 17.4%, 30.5%, and 40.8% at 1 year, 3 years, and 5 years after transplantation, respectively.
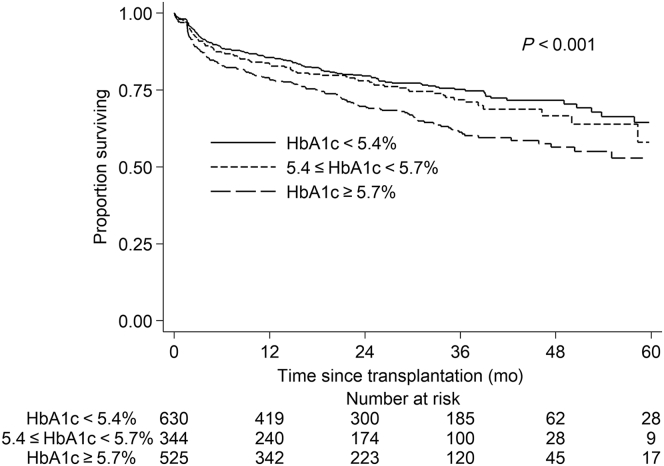
Higher pretransplantation HbA1c was significantly associated with higher incidence of PTDM across the 3 groups (i.e., HbA1c < 5.4% vs. 5.4 ≤ HbA1c < 5.7% vs. HbA1c ≥ 5.7%) in unadjusted analyses (P < 0.001) (Figure 2). The incidence rates of PTDM in the 3 groups were 11.07, 13.00, and 17.87 events/100 person-years, respectively.
Figure 2.
Kaplan−Meier survival curve for development of posttransplantation diabetes mellitus (PTDM), by pretransplantion HbA1c level.
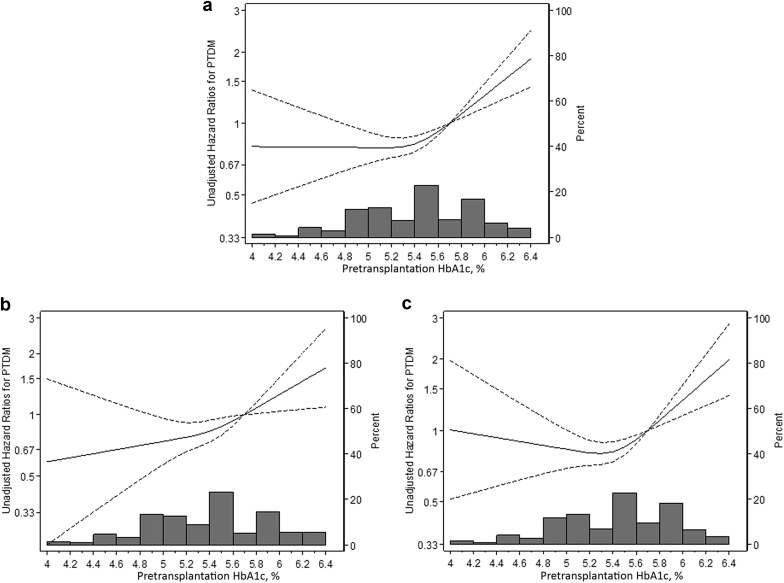
Higher pretransplantation HbA1c was associated with greater risk of PTDM in a nonlinear manner (Figure 3). This nonlinear pattern was seen among both erythropoietin users (Figure 3b) and erythropoietin nonusers (Figure 3c). The goodness of fit was comparable between a restricted cubic spline model with 3 knots and a linear spline model with a single know at 5.4% (likelihood ratio test P = 0.17). HbA1c level was not associated with PTDM below 5.4%, whereas higher HbA1c above this level was strongly associated with PTDM (adjusted HR = 1.84 per 1% higher HbA1c; 95% CI = 1.28, 2.66; P for change in slope = 0.04) (Table 4).
Figure 3.
Unadjusted hazard ratio (solid line) with 95% confidence interval (dashed line) for development of posttransplantation diabetes mellitus (PTDM), by pretransplantation HbA1c. Pretransplantation HbA1c was modeled by restricted cubic splines with 3 knots (5.1, 5.4, and 5.8) in a Cox regression model. The reference value is 5.7% (i.e., cutoff value for prediabetes). (a) All study participants; (b) erythropoietin users; (c) erythropoietin nonusers.
Table 4.
Hazard ratio and 95% confidence interval of PTDM associated with 1% higher level of pretransplantation HbA1c, overall and stratified by erythropoietin use, anemia, induction agent, steroid maintenance, donor type, and components of metabolic syndrome
| Per 1% higher HbA1c | Range of pretransplantation HbA1c level |
P for change in slope | |
|---|---|---|---|
| 4 ≤ HbA1c < 5.4% | 5.4 ≤ HbA1c < 6.5% | ||
| Overall analyses (n = 1499) | |||
| Unadjusted HR (95% CI) | 1.02 (0.69, 1.50) | 2.29 (1.63, 3.22) | 0.01 |
| Adjusted HR (95% CI)a | 0.93 (0.65, 1.35) | 1.84 (1.31, 2.60) | 0.03 |
| Stratified analyses | Adjusted HRa (95% CI) | P-int | Adjusted HRa (95% CI) | P-int | |
|---|---|---|---|---|---|
| Erythropoietin use | |||||
| Yes (n = 532) | 1.29 (0.56, 3.00) | 0.33 | 1.81 (1.05, 3.11) | 0.94 | 0.60 |
| No (n = 967) | 0.80 (0.48, 1.23) | 1.86 (1.16, 2.97) | 0.03 | ||
| Anemiab | |||||
| Absent (n = 705) | 1.22 (0.67, 2.20) | 0.27 | 1.79 (1.00, 3.24) | 0.94 | 0.45 |
| Present (n = 794) | 0.80 (0.46, 1.27) | 1.86 (1.11, 3.10) | 0.05 | ||
| Induction agentc | |||||
| Steroid (n = 1087) | 1.02 (0.65, 1.62) | 0.31 | 1.87 (1.20, 2.93) | 0.34 | 0.12 |
| ATG (n = 699) | 1.34 (0.71, 2.53) | 1.82 (1.11, 2.99) | 0.53 | ||
| IL-2Ra (n = 510) | 1.14 (0.62, 2.08) | 1.70 (0.90, 3.08) | 0.48 | ||
| Steroid maintenance | |||||
| Yes (n = 904) | 1.01 (0.62, 1.64) | 0.43 | 1.79 (1.08, 2.95) | 0.52 | 0.21 |
| No (n = 595) | 0.83 (0.43, 1.60) | 2.18 (1.09, 4.36) | 0.11 | ||
| Donor type | |||||
| Living (n = 948) | 1.13 (0.61, 2.08) | 0.40 | 1.89 (1.16, 3.09) | 0.84 | 0.31 |
| Deceased (n = 551) | 0.75 (0.41, 1.35) | 1.75 (1.00, 3.05) | 0.08 | ||
| Metabolic syndrome | |||||
| Absent (n = 730) | 0.99 (0.55, 1.78) | 0.79 | 1.81 (0.90, 3.64) | 0.85 | 0.29 |
| Present (n = 769) | 0.87 (0.49, 1.55) | 1.95 (1.32, 2.88) | 0.04 | ||
| Body mass index | |||||
| < 30 kg/m2 (n = 1065) | 0.94 (0.55, 1.60) | 0.96 | 1.88 (1.23, 2.87) | 0.94 | 0.09 |
| ≥ 30 kg/m2 (n = 434) | 0.92 (0.48, 1.75) | 1.83 (1.04, 3.24) | 0.19 | ||
| Hypertension | |||||
| Absent (n = 254) | 1.20 (0.50, 2.90) | 0.55 | 1.68 (0.72, 3.91) | 0.75 | 0.66 |
| Present (n = 1245) | 0.88 (0.58, 1.35) | 1.95 (1.35, 2.82) | 0.02 | ||
| Low HDL-cholesterold | |||||
| Absent (n = 552) | 1.00 (0.48, 2.11) | 0.82 | 1.81 (0.89, 3.66) | 0.89 | 0.34 |
| Present (n = 947) | 0.90 (0.56, 1.43) | 1.92 (1.29, 2.86) | 0.03 | ||
| Serum triglyceride level | |||||
| < 150 mg/dl (n = 800) | 0.77 (0.46, 1.29) | 0.24 | 2.12 (1.30, 3.44) | 0.58 | 0.03 |
| ≥ 150 mg/dl (n = 699) | 1.31 (0.70, 2.46) | 1.72 (1.02, 2.90) | 0.58 | ||
Data are hazard ratios with confidence intervals in parentheses, unless otherwise specified.
ATG, antithymocyte globulin; CMV, cytomegalovirus; HCV, hepatitis C virus; HDL, high-density lipoprotein; HLA, human leukocyte antigen; HR, hazard ratio; IL-2Ra, interleukin-2 receptor antagonist; P-int, P-interaction; PTDM, posttransplantation diabetes mellitus; TG, triglyceride.
Adjusted for age, sex, race/ethnicity, history of cardiovascular disease, cause of end-stage renal disease, duration of pretransplantation dialysis, year of transplantation, number of prior transplantations, body mass index, history of hypertension, serum TG and HDL-cholesterol level, blood hemoglobin level, erythropoietin use, HLA mismatch, donor type and age, HCV and CMV serostatus, immunosuppressive agents, and time between date of HbA1c measured and date of transplantation.
Anemia was defined as blood hemoglobin < 11 g/dl.
Not exclusive categories.
Low HDL-cholesterol was defined as < 40 mg/dl in men and < 50 mg/dl in women.
The magnitude of adjusted HR for PTDM per 1% higher HbA1c above 5.4% did not significantly differ by erythropoietin use, presence of anemia, type of induction agent, steroid maintenance use, donor type, presence of the metabolic syndrome, or presence of individual components of the metabolic syndrome, although the sample size of subgroups limits the precision of these estimates (Table 4). The results were also similar across age groups (P-interaction = 0.71 and 0.30 for slope below and above 5.4%, respectively) and race categories (P-interaction = 0.80 and 0.30 for slope below and above 5.4%, respectively).
HbA1c ≥ 5.4% as a Dichotomous Variable and Risk of PTDM
The incidence rate of PTDM was approximately 1.4 times higher among patients who had HbA1c ≥ 5.4% than among those who had HbA1c < 5.4% (P < 0.001) (Table 5). HbA1c ≥ 5.4% remained associated with PTDM after adjustment for demographic factors (adjusted HR = 1.33; 95% CI = 1.08, 1.64). Additional adjustment for components of the metabolic syndrome and for other baseline variables did not substantially change the estimates.
Table 5.
Incidence rate and hazard ratio (95% confidence interval) of PTDM associated with pretransplant HbA1c≥5.4%
| HbA1c < 5.4% | HbA1c ≥ 5.4% | Overall | |
|---|---|---|---|
| Patients, n | 630 | 869 | 1499 |
| Events | 137 | 258 | 395 |
| Incidence rate, per 100 person-years | 11.07 | 15.85 | 13.78 |
| Unadjusted hazard ratio | 1.0 (reference) | 1.42 (1.15, 1.75) | − |
| Partially adjusted hazard ratio | |||
| Model 1a | 1.0 (reference) | 1.33 (1.07, 1.64) | − |
| Model 2b | 1.0 (reference) | 1.31 (1.06, 1.62) | − |
| Model 3c | 1.0 (reference) | 1.32 (1.07, 1.64) | − |
| Fully adjustedd hazard ratio | 1.0 (reference) | 1.28 (1.03, 1.58) | − |
CMV, cytomegalovirus; HCV, hepatitis C virus; HDL, high-density lipoprotein; HLA, human leukocyte antigen; PTDM, posttransplantation diabetes mellitus; TG, triglyceride.
Model 1: adjusted for age, sex, and race/ethnicity.
Model 2: adjusted for age, sex, race/ethnicity, and components of metabolic syndrome (history of hypertension, body mass index, and serum TG and HDL-cholesterol level).
Model 3: adjusted for all covariates except components of metabolic syndrome in fully adjusted model.
Adjusted for age, sex, race/ethnicity, history of cardiovascular disease, cause of end-stage renal disease, duration of pretransplantation dialysis, year of transplantation, number of prior transplantations, body mass index, history of hypertension, serum TG and HDL-cholesterol level, blood hemoglobin level, erythropoietin use, HLA mismatch, donor type and age, HCV and CMV serostatus, immunosuppressive agents, and time between date of HbA1c measured and date of transplantation.
The association between HbA1c ≥ 5.4% and risk of PTDM was similar across erythropoietin use (adjusted HR = 1.32; 95% CI = 0.93, 1.87) and 1.25 (0.96, 1.63) among those receiving and those not receiving erythropoietin, respectively; P-interaction = 0.82). The results were also similar in those with anemia (adjusted HR = 1.31; 95% CI = 0.99, 1.75) and those without anemia (adjusted HR = 1.21; 95% CI = 0.88, 1.67; P-interaction = 0.71). The strength of the association between HbA1c ≥ 5.4% and PTDM did not differ by type of induction agent, steroid maintenance use, donor type, presence of the metabolic syndrome, or presence of individual components of the metabolic syndrome, although the sample size of subgroups limits the precision of these estimates (Table 6). The results were also consistent across age groups (P-interaction = 0.40) and race categories (P-interaction = 0.40).
Table 6.
Adjusted hazard ratio (95% confidence interval) of PTDM for pretransplantation HbA1c ≥ 5.4%, overall and stratified by erythropoietin use, anemia, induction agent, steroid maintenance, donor type, and components of metabolic syndrome
| n | Adjusteda hazard ratio | P interaction | |
|---|---|---|---|
| Overall | 1499 | 1.28 (1.03, 1.58) | – |
| Erythropoietin use | |||
| Yes | 532 | 1.32 (0.90, 1.93) | 0.84 |
| No | 967 | 1.25 (0.95, 1.66) | |
| Anemiab | |||
| Absent | 705 | 1.21 (0.88, 1.67) | 0.70 |
| Present | 794 | 1.31 (0.99, 1.75) | |
| Induction agentc | |||
| Steroid | 1087 | 1.33 (1.01, 1.75) | 0.70 |
| ATG | 699 | 1.46 (0.98, 2.17) | |
| IL-2Ra | 510 | 1.29 (0.92, 1.81) | |
| Steroid maintenance | |||
| Yes | 904 | 1.39 (1.10, 1.77) | 0.62 |
| No | 595 | 1.23 (0.78, 1.92) | |
| Donor type | |||
| Living | 948 | 1.30 (0.99, 1.70) | 0.77 |
| Deceased | 551 | 1.23 (0.89, 1.69) | |
| Metabolic syndrome | |||
| Absent | 730 | 1.20 (0.87, 1.62) | 0.61 |
| Present | 769 | 1.34 (0.96, 1.87) | |
| Body mass index | |||
| < 30 kg/m2 | 1065 | 1.31 (0.99, 1.72) | 0.88 |
| ≥ 30 kg/m2 | 434 | 1.26 (0.90, 1.77) | |
| Hypertension | |||
| Absent | 254 | 1.34 (0.80, 2.24) | 0.85 |
| Present | 1245 | 1.26 (1.98, 1.62) | |
| Low HDL-cholesterold | |||
| Absent | 552 | 1.15 (0.77, 1.71) | 0.63 |
| Present | 947 | 1.30 (1.00, 1.70) | |
| Serum triglyceride level | |||
| < 150 mg/dl | 800 | 1.25 (0.95, 1.64) | 0.68 |
| ≥ 150 mg/dl | 699 | 1.35 (0.99, 1.84) |
ATG, antithymocyte globulin; CMV, cytomegalovirus; HCV, hepatitis C virus; HDL, high-density lipoprotein; HLA, human leukocyte antigen; HR, hazard ratio; IL-2Ra, interleukin-2 receptor antagonist; PTDM, posttransplantation diabetes mellitus; TG, triglyceride.
Adjusted for age, sex, race/ethnicity, history of cardiovascular disease, cause of end-stage renal disease, duration of pretransplantation dialysis, year of transplantation, number of prior transplantations, body mass index, history of hypertension, serum TG and HDL-cholesterol level, blood hemoglobin level, erythropoietin use, HLA mismatch, donor type and age, HCV and CMV serostatus, immunosuppressive agents, and time between date of HbA1c measured and date of transplantation.
Anemia was defined as blood hemoglobin < 11 g/dl.
Not exclusive categories.
Low HDL-cholesterol was defined as < 40 mg/dl in men and < 50 mg/dl in women.
Sensitivity Analyses
Higher HbA1c above 5.4% was strongly associated with PTDM in analyses starting follow-up at 45 days after transplantation (356 PTDM events, adjusted HR = 1.86 per 1% higher HbA1c; 95% CI = 1.27, 2.74). Likewise, results were materially unchanged in analyses with a dichotomous HbA1c variable (adjusted HR associated with HbA1c ≥ 5.4% = 1.30; 95% CI = 1.03, 1.62). Higher HbA1c above 5.4% remained significantly associated with PTDM in analyses starting follow-up at 1 year after transplantation (148 PTDM events, adjusted HR = 2.96 per 1% higher HbA1c; 95% CI = 1.64, 5.36). Similarly, HbA1c ≥ 5.4% remained strongly associated with greater risk of PTDM (adjusted HR = 1.38; 95% CI = 1.00, 1.98) in analyses starting follow-up at 1 year after transplantation. Results were similar in analyses limited to those patients whose HbA1c was measured within 1 year after transplantation (n = 1079, 274 PTDM events).
Discussion
In this analysis of 1499 primary kidney transplant recipients, HbA1c measured before transplantation was independently associated with the risk of PTDM. Higher pretransplantation HbA1c was associated with greater risk of PTDM at HbA1c levels above 5.4%, with an 84% higher risk associated with each 1% higher HbA1c above this level. HbA1c level was not associated with PTDM at levels below 5.4%. Recipients with HbA1c ≥ 5.4% had a 31% higher risk of PTDM than their counterparts with HbA1c < 5.4%. The observed associations were independent of potential confounders, were seen in both erythropoietin users and nonusers and those with and without anemia, and were similar across type of induction agent, steroid maintenance use, donor type, or metabolic risk groups. The novel finding of our study is that higher pretransplantation HbA1c was associated with greater risk of PTDM in a nonlinear fashion. Our findings suggest that the increase in risk for PTDM starts at 5.4%, which is well below the current threshold of 5.7% for identifying prediabetes in the general population.
The cumulative incidence of PTDM in our study (17.4% and 30.5% at 1 year and 3 years, respectively) is similar to results from previous studies.1, 2, 18 Recipients are at greatest risk for PTDM within the first 6 months after transplantation, with most cases occurring within the first year.19, 20 Of 395 PTDM events over the 5-year follow-up period in our study, 62.5% events occurred within the first year.
Tatar et al.21 reported an adjusted odds ratio of 4.6 for PTDM in the first year after transplantation per 1% higher pretransplantation HbA1c in a study of 204 Turkish kidney transplant recipients (54 cases of PTDM). Conversely, Tokodai et al.22 did not show a statistically significant association between pretransplantation HbA1c and PTDM in a study of 119 Japanese recipients (17 cases of PTDM) (adjusted odds ratio = 3.09; 95% CI = 0.90, 11.1; P = 0.07). Interestingly, a significant association between pretransplantation HbA1c and PTDM was observed only among the subgroup of recipients receiving no or low-dose erythropoietin (n = 85, adjusted odds ratio = 9.18 per 1% higher HbA1c (95% CI = 1.64, 64.5; P < 0.05), but no association in 34 recipients on higher doses (data not reported). The authors speculated that the lack of association might be due to a high degree of variability in HbA1c levels in patients receiving high-dose erythropoietin.22 However, as the sample size of high-dose erythropoietin users was only 34, the lack of association could be related to the lack of statistical power as well. Moreover, Tokodai et al.22 did not formally test whether there existed an interaction between pretransplantation HbA1c and erythropoietin use (no or low-dose vs. high-dose) with regard to risk of PTDM. Erythropoietin dosage and blood hemoglobin level influence HbA1c level in patients with end-stage renal disease.23 Neither of these 2 studies adjusted for blood hemoglobin level in the analyses, whereas it was included as a covariate in our study. The strength of the associations between pretransplantation HbA1c and PTDM did not differ significantly by erythropoietin use or presence of anemia in our study. This might be explained by our sample having similar mean HbA1c between erythropoietin users and nonusers (5.40 vs. 5.45%, respectively, P = 0.07) and those with anemia and without anemia (5.42 vs. 5.45%, respectively, P = 0.11).
Optimal cut-off values of HbA1c for diagnosing PTDM remain uncertain. According to the International Consensus Meeting Report on posttransplantation diabetes mellitus, HbA1c-based diagnosis of PTDM is recommended while oral glucose tolerance test is still considered as the gold standard.9 However, careful interpretation must be made regarding its use in the early posttransplantation period, as a normal HbA1c will not exclude diagnosis of PTDM in the presence of posttransplantation anemia and/or dynamic renal allograft function.9 Shabir et al.24 suggested separate cut-off values of HbA1c for diagnosing PTDM (i.e., 6.2% and 6.5% (equivalent to the general population) at 3 months and beyond 3 months, respectively).
Higher HbA1c before transplantation remained a strong predictor for PTDM events occurring after 45 days or 1 year after transplantation in our study. This approach minimized the possibility of claiming undiagnosed diabetes before transplantation as PTDM or diagnosing transient posttransplantation hyperglycemia as PTDM. Moreover, these findings emphasize that pretransplantation HbA1c, as a marker of intrinsic abnormalities in glucose metabolism, is an important predictor for PTDM, independent of specific risk factors related to transplantation, such as immunosuppression.
HbA1c testing is well standardized, shows low intraindividual variation, is the most reliable indicator of glycemic exposure over the recent 2-3 months, and is associated with long-term complications in the general population.10 Moreover, it does not require fasting or restriction to a certain time of waiting. Although HbA1c is less reliable in patients with severely impaired kidney function than in the general population,12, 25, 26 higher HbA1c level is associated with higher risk of mortality among diabetic patients on dialysis.27, 28, 29 Furthermore, higher HbA1c is associated with greater risk of mortality among nondiabetic patients with non−dialysis-dependent chronic kidney disease,30 as well as among nondiabetic patients on hemodialysis.31 Therefore, HbA1c could also be a useful tool for pretransplantation PTDM risk evaluation, based on our findings.
Inherent limitations of the observational study design must be acknowledged. Although our study has a much larger sample size than the previous 2 studies,21, 22 only 4.7% (n = 1499) of potentially eligible recipients (n = 36,855) were included in the analyses. This was mainly because HbA1c was available for only a small proportion of recipients. Despite the 2005 revised CMS Form 2728 requiring health care providers to report HbA1c level regardless of patients’ diabetic status, only 9.4% of nondiabetic patients had an HbA1c level available. No significant improvement has been made in HbA1c measurement between 2005 and 2011. Among a total of 4316 providers who saw 36,855 nondiabetic patients before transplantation, 3420 (79.2%) never reported HbA1c and 73 (1.7%) always reported HbA1c. Providers with a higher proportion of reporting HbA1c on the CMS2728 form were more likely to work in hospital-based and nonprofit facilities (Supplementary Table S2). A total of 75 providers who mostly (proportion of reporting HbA1c = 90%−100%) reported HbA1c in nondiabetic patients were evenly distributed across 18 ESRD networks. However, using our limited data, the question of why a subset of nondiabetic patients were tested for HbA1c was difficult to answer clearly. Nonetheless, the incidence of PTDM between included recipients and excluded recipients was comparable, suggesting that the risk profile for the development of PTDM on average is similar between patients included and those excluded from the study. Therefore, the potential for a spurious association due to selection bias is minimal. Another limitation is that the included recipients were less likely to be black (13.9% vs. 17.8%) than the excluded recipients. Despite a higher risk of PTDM in black patients in comparison to white patients,20 black patients were less likely to be tested for HbA1c in our study. Similarly, previous studies reported that lower receipt of HbA1c testing in black diabetic than in white diabetic patients.32, 33 Considering the fact that HbA1c levels are higher in blacks than in whites independent of level of glycemia,34, 35 and that blacks are at higher risk for PTDM than whites,20 this limitation would tend to dilute the strength of the association between HbA1c and PTDM. Therefore, the true association between HbA1c and PTDM may be stronger than the observed association with a limited sample in our study. Although we excluded diabetic patients using multiple sources (i.e., CMS Form 2728, United Network for Organ Sharing transplant candidate registration form, or USRDS Medicare claims data), there could be undiagnosed diabetic recipients included in our study. However, consistent results with analyses starting follow-up at 1 year after transplantation (i.e., analyses only for those who had not developed PTDM until 1 year after transplantation) suggest that the observed association is not likely to be explained by inclusion of undiagnosed diabetes with higher HbA1c before transplantation. Our study is further limited by outome ascertainments relying on the USRDS Medicare claims data. Approximately 27% of nondiabetic recipients in the USRDS data were excluded because of a lack of Medicare claims data. Individuals having another insurance (e.g., private insurance) simultaneously with Medicare coverage during the posttransplantation period may not be included in USRDS Medicare claims data. This limitation could potentially cause underascertainment of PTDM. However, the incidence of PTDM in our study (i.e., 17.4% at 1 year after transplantation) is consistent with data from the USRDS and a French study reporting a 16% and 18% incidence of PTDM at 1 year after transplantation, respectively.1, 18 Although we took into account erythropoietin use before kidney transplantation in our analyses, we could not account for the dosage of erythropoietin, as theses data are unavailable. Moreover, there might be some degree of misclassification of erythropoietin use at the time of measured HbA1c because of the lack of prescription date for erythropoietin. In addition, we could not evaluate whether HbA1c adds predictive power to fasting blood glucose, because of a lack of data. Finally, despite multivariate adjustment for known potential confounders, there are additional potential confounders (e.g., family history of diabetes and change in BMI after transplantation) for which we could not adjust that may explain the observed associations. Despite these limitations, this study improves current understanding of how pretransplantation HbA1c is associated with PTDM among kidney transplant recipients.
In conclusion, we found that higher HbA1c before transplantation is independently associated with greater risk of PTDM, and that the increase in risk associated with higher HbA1c starts at levels above 5.4%, well below current thresholds for prediabetes. These findings suggest that HbA1c testing could be a useful screening tool as a part of a pretransplantation evaluation to identify recipients at greatest risk for PTDM. This may help guide monitoring and treatment strategies (e.g., immunosuppression and lifestyle modification) to prevent or delay the onset of PTDM.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The project described was supported by grant number T32 HL007024 from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
Table S1. Comparison of characteristics and incidence of posttransplantation diabetes mellitus between nondiabetic recipients with and without HbA1c before transplantation.
Table S2. Provider characteristics by the proportion of reporting HbA1c on CMS2728 form among nondiabetic patients (total N of providers = 4316).
Supplementary material is linked to the online version of the paper at http://www.kireports.org.
Supplementary Material
Comparison of characteristics and incidence of posttransplantation diabetes mellitus between nondiabetic recipients with and without HbA1c before transplantation.
Provider characteristics by the proportion of reporting HbA1c on CMS2728 form among nondiabetic patients (total N of providers = 4316).
References
- 1.Kasiske B.L., Snyder J.J., Gilbertson D. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 2.Woodward R.S., Schnitzler M.A., Baty J. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant. 2003;3:590–598. doi: 10.1034/j.1600-6143.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 3.Wauters R.P., Cosio F.G., Suarez Fernandez M.L. Cardiovascular consequences of new-onset hyperglycemia after kidney transplantation. Transplantation. 2012;94:377–382. doi: 10.1097/TP.0b013e3182584831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharif A., Baboolal K. Complications associated with new-onset diabetes after kidney transplantation. Nat Rev Nephrol. 2012;8:34–42. doi: 10.1038/nrneph.2011.174. [DOI] [PubMed] [Google Scholar]
- 5.Yates C.J., Fourlanos S., Hjelmesaeth J. New-onset diabetes after kidney transplantation—changes and challenges. Am J Transplant. 2012;12:820–828. doi: 10.1111/j.1600-6143.2011.03855.x. [DOI] [PubMed] [Google Scholar]
- 6.Hornum M., Jørgensen K.A., Hansen J.M. New-onset diabetes mellitus after kidney transplantation in Denmark. Clin J Am Soc Nephrol. 2010;5:709–716. doi: 10.2215/CJN.05360709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosio F.G., Kudva Y., van der Velde M. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67:2415–2421. doi: 10.1111/j.1523-1755.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 8.Bergrem H.A., Valderhaug T.G., Hartmann A. Glucose tolerance before and after renal transplantation. Nephrol Dial Transplant. 2010;25:985–992. doi: 10.1093/ndt/gfp566. [DOI] [PubMed] [Google Scholar]
- 9.Sharif A., Hecking M., de Vries A.P.J. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14:1992–2000. doi: 10.1111/ajt.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werzowa J., Hecking M., Haidinger M. The diagnosis of posttransplantation diabetes mellitus: meeting the challenges. Curr Diab Rep. 2015;15:27. doi: 10.1007/s11892-015-0601-x. [DOI] [PubMed] [Google Scholar]
- 13.Hebert P.L., Geiss L.S., Tierney E.F. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14:270–277. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 14.Grundy S.M., Brewer H.B., Cleeman J.I. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–e18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 15.Alberti K.G.M.M., Zimmet P., Shaw J., IDF Epidemiology Task Force Consensus Group The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 16.Sun X., Du T., Huo R. Impact of HbA1c criterion on the definition of glycemic component of the metabolic syndrome: the China Health and Nutrition Survey 2009. BMC Public Health. 2013;13:1045. doi: 10.1186/1471-2458-13-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharif A., Cohney S. Post-transplantation diabetes—state of the art. Lancet Diabetes Endocrinol. 2016;4:337–349. doi: 10.1016/S2213-8587(15)00387-3. [DOI] [PubMed] [Google Scholar]
- 18.Augusto J.-F., Subra J.-F., Duveau A. Relation between pretransplant magnesemia and the risk of new onset diabetes after transplantation within the first year of kidney transplantation. Transplantation. 2014;97:1155–1160. doi: 10.1097/01.TP.0000440950.22133.a1. [DOI] [PubMed] [Google Scholar]
- 19.Abbott K.C., Lentine K.L., Bucci J.R. Impact of diabetes and hepatitis after kidney transplantation on patients who are affected by hepatitis C virus. J Am Soc Nephrol. 2004;15:3166–3174. doi: 10.1097/01.ASN.0000145439.48387.BF. [DOI] [PubMed] [Google Scholar]
- 20.Ghisdal L., Van Laecke S., Abramowicz M.J. New-onset diabetes after renal transplantation: risk assessment and management. Diabetes Care. 2012;35:181–188. doi: 10.2337/dc11-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatar E., Kircelli F., Demirci M.S. Pre-transplant HbA1c level as an early marker for new-onset diabetes after renal transplantation. Int Urol Nephrol. 2013;45:251–258. doi: 10.1007/s11255-012-0304-z. [DOI] [PubMed] [Google Scholar]
- 22.Tokodai K., Amada N., Haga I. Pretransplant HbA1c is a useful predictor for the development of new-onset diabetes in renal transplant recipients receiving no or low-dose erythropoietin. Int J Endocrinol. 2014;2014:436725. doi: 10.1155/2014/436725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uzu T., Hatta T., Deji N. Target for glycemic control in type 2 diabetic patients on hemodialysis: effects of anemia and erythropoietin injection on hemoglobin A(1c) Ther Apher Dial. 2009;13:89–94. doi: 10.1111/j.1744-9987.2009.00661.x. [DOI] [PubMed] [Google Scholar]
- 24.Shabir S., Jham S., Harper L. Validity of glycated haemoglobin to diagnose new onset diabetes after transplantation. Transpl Int. 2013;26:315–321. doi: 10.1111/tri.12042. [DOI] [PubMed] [Google Scholar]
- 25.Bunn H.F., Haney D.N., Kamin S. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J Clin Invest. 1976;57:1652–1659. doi: 10.1172/JCI108436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coban E., Ozdogan M., Timuragaoglu A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004;112:126–128. doi: 10.1159/000079722. [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K., Kopple J.D., Regidor D.L. A1C and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30:1049–1055. doi: 10.2337/dc06-2127. [DOI] [PubMed] [Google Scholar]
- 28.Ricks J., Molnar M.Z., Kovesdy C.P. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes. 2012;61:708–715. doi: 10.2337/db11-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J.I., Bae E., Kim Y.-L. Glycemic control and mortality in diabetic patients undergoing dialysis focusing on the effects of age and dialysis type: a prospective cohort study in Korea. PloS One. 2015;10:e0136085. doi: 10.1371/journal.pone.0136085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trivin C., Metzger M., Haymann J.-P. Glycated hemoglobin level and mortality in a nondiabetic population with CKD. Clin J Am Soc Nephrol. 2015;10:957–964. doi: 10.2215/CJN.08540814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ok E.S., Asci G., Toz H. Glycated hemoglobin predicts overall and cardiovascular mortality in non-diabetic hemodialysis patients. Clin Nephrol. 2014;82:173–180. doi: 10.5414/cn108251. [DOI] [PubMed] [Google Scholar]
- 32.LeMaster J.W., Chanetsa F., Kapp J.M. Racial disparities in diabetes-related preventive care: results from the Missouri Behavioral Risk Factor Surveillance System. Prev Chron Dis. 2006;3:A86. [PMC free article] [PubMed] [Google Scholar]
- 33.Gary T.L., McGuire M., McCauley J. Racial comparisons of health care and glycemic control for African American and white diabetic adults in an urban managed care organization. Dis Manag. 2004;7:25–34. doi: 10.1089/109350704322918970. [DOI] [PubMed] [Google Scholar]
- 34.Ziemer D.C., Kolm P., Weintraub W.S. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152:770–777. doi: 10.7326/0003-4819-152-12-201006150-00004. [DOI] [PubMed] [Google Scholar]
- 35.Saaddine J.B., Fagot-Campagna A., Rolka D. Distribution of HbA(1c) levels for children and young adults in the U.S.: Third National Health and Nutrition Examination Survey. Diabetes Care. 2002;25:1326–1330. doi: 10.2337/diacare.25.8.1326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of characteristics and incidence of posttransplantation diabetes mellitus between nondiabetic recipients with and without HbA1c before transplantation.
Provider characteristics by the proportion of reporting HbA1c on CMS2728 form among nondiabetic patients (total N of providers = 4316).