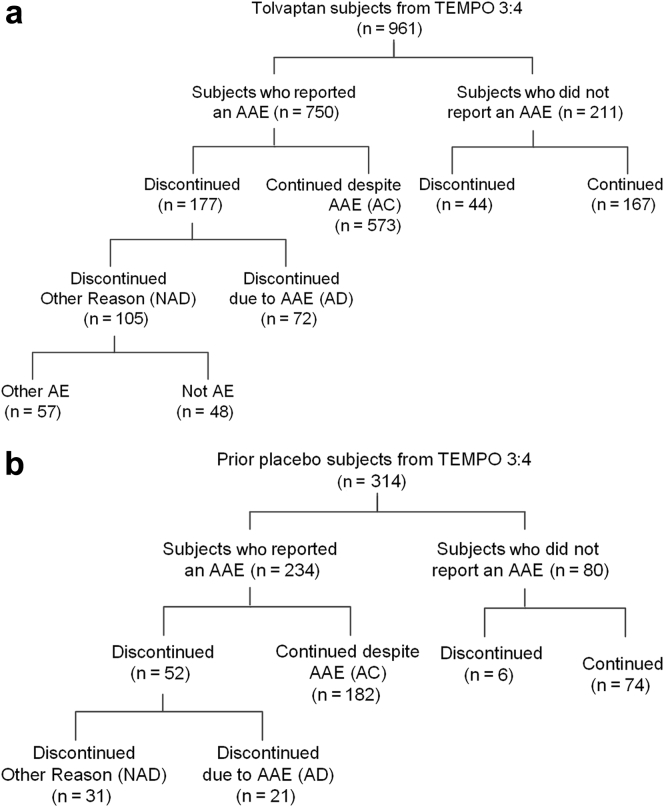
Figure 1.
Flow chart of tolvaptan subjects into categories of participants who reported or did not report an aquaretic adverse event (AAE). (a) Tolvaptan subjects in the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) 3:4 trial. (b) Prior placebo subjects in TEMPO 4:4. AC, aquaretic-continued; AD, aquaretic-discontinued; AE, adverse event; NAD, non–aquaretic discontinued.