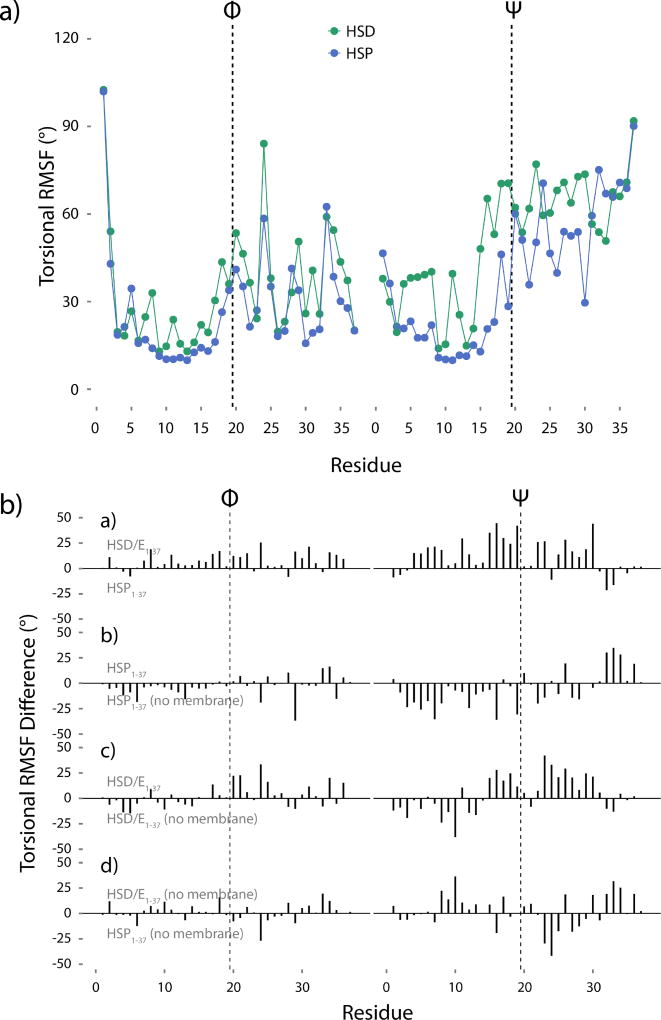
Figure 7.
a) Torsional RMSF of the hIAPP1–37 HMMM backbone Φ and Ψ angles. b) Torsional RMSF differences. If the difference is positive, the top peptide is more flexible, whereas a negative difference indicates more flexibility in the bottom peptide. The difference between HMMM-bound HSD/E1–37 and HMMM-bound HSP1–37 shows the effect of protonating His18. The difference between HMMM-bound HSP1–37 and solution HSP1–37 shows the effect of the membrane on the HSP1–37 peptide. The difference between HMMM-bound HSD/E1–37 and solution HSD/E1–37 shows the effect of the membrane on the HSD/E1–37 peptide. The difference between solution HSD/E1–37 and solution HSP1–37 shows the effect of low pH on the free peptide. The first 20 ns of simulation have not been included in the analysis. The vertical dashed lines in all panels indicate the divide between the 19 N-terminal residues and the 18 C-terminal residues.