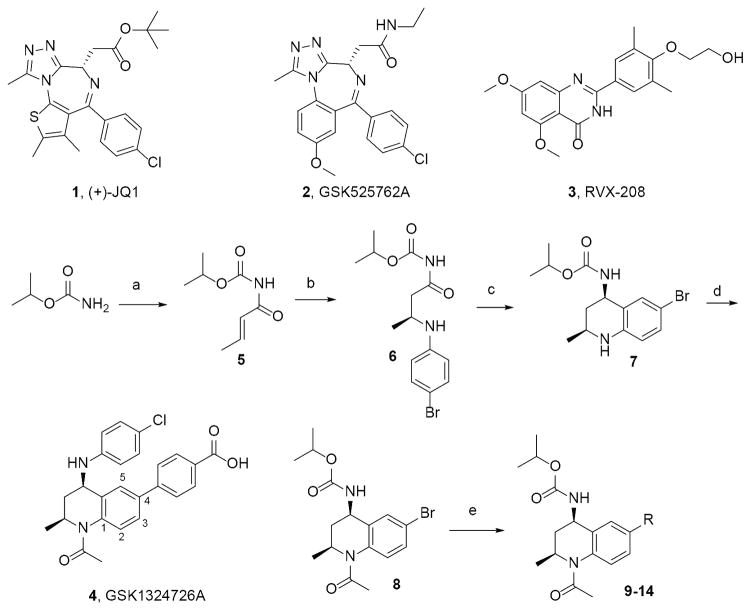
Figure 1. Exemplar BETi and synthetic scheme for THQ analogs 9–14.
Reagents and conditions: a) crotonoyl chloride, LiHMDS, THF −78 °C; b) 4-bromo-aniline, [(R)-(+)-2,2′-bis(diphenylphosphino)-1,1′binaphthyl]-diaquo-Pd(II) bis(triflate), toluene, RT; c) NaBH4, MgCl2, EtOH −10 °C; d) AcCl, DIEPA, CH2Cl2 0 °C; e) R-B(OH)2, K3PO4, BrettPhos Palladacycle Gen. 3, BrettPhos, 2-methyl-2-butanol, 100 °C, 20–89% yield range. See Supporting Information (SI) for specific structures and details.