Abstract
Introduction
Fibroblast growth factor 23 (FGF23) could contribute to cardiovascular morbidity in chronic kidney disease. In studies of kidney transplant recipients, a high circulating level of FGF23 has been associated with death and graft loss independently of estimated glomerular filtration rate (GFR). Whether FGF23 is associated with adverse outcomes in the early posttransplantation period is unknown.
Methods
We analyzed a cohort of 845 kidney transplant recipients in stable condition who had GFR measured in the first years after transplantation with a median follow-up of 71 months.
Results
A high FGF23 concentration was associated with death or graft loss in univariate analysis, but this association was lost after adjustment for measured GFR. In contrast, FGF23 remained significantly associated with the composite outcome when estimated GFR was substituted for measured GFR. We also observed that follow-up duration modified the association between FGF23 and outcome. Although FGF23 was not associated with any endpoint in the full duration of the study, we found an independent association between FGF23 and the incidence of graft loss within the 4 years after FGF23 measurement. We did not find an association between FGF23 levels and left ventricular mass in a subgroup of 227 patients who had echocardiography performed within 3 months of FGF23 measurement.
Discussion
This study demonstrates that FGF23 measured during the first year after transplantation is not an independent predictor of death and graft loss and is not associated with left ventricular hypertrophy in the posttransplantation period. It further unveils important factors modifying the association between FGF23 and outcome in this population.
Keywords: fibroblast growth factor 23, glomerular filtration rate, graft loss, mortality, transplant
Kidney transplantation is considered the preferred treatment for end-stage renal disease. Compared with dialysis, renal transplantation is associated with better survival and quality of life.1 Although important advances have been made in surgery and immunosuppression regimens, the overall mortality associated with renal transplantation has remained stable while graft survival has improved.2 Mortality and early graft loss remain significant concerns. It is therefore of primary importance to elucidate the factors that are implicated in and/or able to predict death and graft loss in transplant recipients, particularly at an early stage after transplantation.
Fibroblast growth factor 23 (FGF23) is an endocrine polypeptide belonging to the fibroblast growth factor (FGF) family.3 In contrast to most FGFs, which exert only paracrine and intracrine signaling, FGF23 is released in the systemic circulation and acts as a hormone. It binds to a receptor complex associated with a FGF receptor and α-Klotho, its specific co-receptor. FGF23 controls phosphate homeostasis,4 and in the kidney, it increases urinary phosphate excretion by reducing the membrane expression of the sodium phosphate co-transporters NPT2a and NPT2c. It also reduces the net production of 1,25-dihydroxyvitamin D by proximal tubular cells, both by reducing its conversion from 25-hydroxyvitamin D through the 1α-hydroxylase CYP27B1 and by increasing its catabolism by the 24-hydroxylase CYP24A1. In the absence of advanced renal insufficiency, FGF23 also acts on parathyroid cells to reduce the production of parathyroid hormone (PTH).5 In chronic kidney disease (CKD), FGF23 levels increase with the reduction of the glomerular filtration rate (GFR) to prevent phosphate retention secondary to nephron reduction.6 This adaptive response plays an important role in the reduction of 1,25-dihydroxyvitamin D concentrations observed in patients with CKD and contributes to secondary hyperparathyroidism.7
The FGF23 plasma concentration is an independent predictor of death in patients with CKD undergoing dialysis.8, 9 The mechanisms involved have remained elusive so far, but recent evidence has suggested a link between FGF23 and left ventricle hypertrophy and dysfunction in patients with CKD.10 Exogenous FGF23 has been shown to induce cardiomyocyte hypertrophy in vitro and in vivo, whereas inhibition of FGF signaling was found to reduce cardiac hypertrophy in a rodent model of CKD.10, 11 More recently, a high FGF23 level has been associated with an increased risk of hospitalization related to infection in patients undergoing hemodialysis. In rodents, high FGF23 plasma levels have been shown to impair neutrophil recruitment at bacterial infection sites.12, 13
To date, 2 studies have linked FGF23 to mortality and graft loss in renal transplant recipients.14, 15 In these studies, most of the patients were included several years after transplantation: the median transplantation vintage at inclusion was 7 and 6.2 years in studies by Wolf et al. and Baia et al., respectively.14, 15 These studies provided no information on the association between FGF23 and outcomes during the first years after renal transplantation. Furthermore, patients who lost grafts or died during the first year after transplantation were not studied. To date, no data have been published regarding the association between FGF23 measured in the first year after renal transplantation and later outcomes.
Since 2005, the standard care for kidney transplant recipients at our institution has included a measurement of GFR by iohexol clearance and an assessment of FGF23 in the first year after transplantation. We took advantage of this monitoring requirement to assess the link between FGF23 and posttransplantation outcome.
Methods
Study Population
All patients received transplants and were followed up in the Department of Renal Transplantation at Necker Hospital, Paris, France. Since September 2005, the standard longitudinal care for kidney transplant recipients has included a measurement of GFR and mineral metabolism parameters 1 year after transplantation and every 2 years thereafter. From October 2005 to April 2012, all patients who were evaluated at least 10 months after transplantation were included in the study. Patients not evaluated in the first year for GFR measurement and FGF23 (generally because of complications requiring hospitalization) were included at a subsequent evaluation up to 38 months after transplantation.
Data Collection
All data were prospectively collected by the physician in charge of the patient in the cross-validated French database DIVAT (Données Informatisées et VAlidées en Transplantation, http://www.divat.fr/). The DIVAT database has been approved by the National French Commission for Bioinformatics Data and Patient Liberty (registration number 1016618). The collected data, which are computerized in real time and at each transplant anniversary, include information concerning:
-
•
the donor (age, gender, deceased or living donor, cold ischemia time)
-
•
the recipient (age, gender, primary etiology of CKD, previous duration of dialysis)
-
•
the transplantation procedure
-
•
the immune suppression regimen
-
•
the occurrence of delayed graft function (defined as the need for hemodialysis during the first week after transplantation)
-
•
graft failure (defined as a re-transplantation or a return to long-term dialysis)
The information entered in the DIVAT database is audited every year. In addition, we prospectively collected data for height, body weight, and blood pressure at the time of GFR and FGF23 measurement.
Biochemistry
FGF23 plasma concentration was measured with an enzyme-linked immunosorbent assay (Immutopics, Inc, San Clemente, CA) that recognizes the C-terminal part of the peptide. Serum PTH concentration was measured with an immunochemiluminescent assay performed on the Elecsys analyzer (Roche Diagnostics, Meylan, France). Total calcium, ionized calcium, and phosphate and creatinine concentrations were measured using standard methods. Calcitriol and 25-hydroxyvitamin D concentrations were measured with a radioimmunoassay (DiaSorin, Stillwater, MN) and a radioimmunoassay after immunoextraction (IDS Ltd, Boldon, UK), respectively.
GFR was determined by iohexol clearance or inulin clearance when allergy to iohexol was suspected on the basis of patient history as previously described.16 The Modification of Diet in Renal Disease (MDRD) 3-variable equation was used to determine estimated GFR (eGFR).17 Maximal tubular reabsorption of phosphate/GFR was calculated in the fasting state in the morning.
Echocardiography
All patients had transthoracic echocardiography performed in our cardiology center (Adult Cardiology Functional Unit, Hôpital Necker-Enfants Malades) by trained cardiologists. Data were acquired from standard parasternal and apical 4-chamber views. Left ventricular mass was calculated with ultrasound guidance from the parasternal long-axis view and was normalized for body surface area to estimate the left ventricular mass index. Fraction of shortening and left ventricular ejection fraction were then calculated.
Statistical Analyses
Categorical data were described as numbers (%) and continuous variables as mean (SD) or median (first quartile [Q1], third quartile [Q3]) as appropriate. We systematically considered FGF23 measured at first evaluation after transplantation as a quantitative variable that we log-transformed for statistical analysis whenever possible and as a categorized variable according to tertiles. We compared patient characteristics according to tertiles of FGF23 using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables.
A Bland and Altman plot was created to measure agreement between measured GFR (mGFR) and eGFR. The y-axis represents the difference between the 2 measures, and the x-axis represents the mean of the 2 measures. The lines indicate a difference of 0 and the limits of agreement.
We aimed to determine whether FGF23 was independently associated with death or graft failure and explored possible variations in this association. We performed 2 main analyses: 1 based on a short period (follow-up until 4 years after the initial renal evaluation) and 1 based on a long period (median follow-up duration: 66 months [Q1–Q3: 47–89]). For each of these analyses, we used Cox regression models with adjustment for mGFR only and full adjustment for variables associated with death or graft failure in univariate analysis or considered as important in the literature (i.e., mGFR, age at inclusion, duration of dialysis, transplantation rank, type of donor [dead or alive], delayed graft function, systolic blood pressure, phosphate, logPTH, logcalcitriol). For GFR, we did a sensitivity analysis using eGFR instead of mGFR. The hypothesis of proportional hazards was systematically evaluated. For long-term follow-up, we had to use FGF23 instead of logFGF23 because the hypothesis of proportional hazards was not verified with logFGF23.
As secondary analyses, we assessed whether FGF23 was associated with each event separately: occurrence of graft failure and death for the short-term follow-up. For death, this analysis was based on multivariate Cox regression models adjusted for mGFR only and for mGFR and other variables. To evaluate whether FGF23 was associated with graft failure, we used 2 different models: a cause-specific Cox model and a Fine and Gray model, taking into account the competing risk of death. For both models, we adjusted for mGFR only and for mGFR and other variables. Both models provided consistent results.
All tests were 2-sided, and a P value <0.05 was considered significant. Our analysis was exploratory, aimed at better understanding factors associated with variation in the association between FGF23 and outcome. Therefore we did not correct alpha risk for multiple statistical tests. Statistical analyses were performed with R version 3.2.3 (http://www.R-project.org/), a statistical computing program.18
Results
Study Population
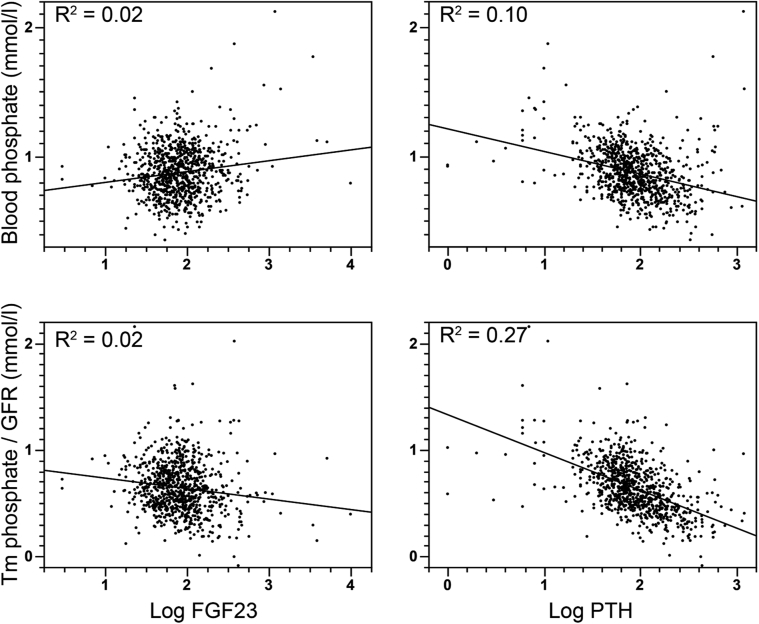
From October 2005 to April 2012, 845 kidney transplant recipients in stable condition had measurements of FGF23 and mGFR obtained. The characteristics of these patients are shown in Table 1. In agreement with our systematic monitoring policy, the median transplantation vintage of patients at the time of FGF23 and GFR measurements was 12 months (Q1–Q3: 12–13). These measurements were obtained from 87% and 92% of the patients within the first 2 and 3 years after transplantation, respectively. Fifty-eight percent of the patients were men. The mean age at inclusion was 49 ± 14 years. The median mGFR was 54 ml/min per 1.73m2 (Q1–Q: 342–64), and median FGF23 concentration was 78 RU/ml (Q1–Q3: 50–121). Only 28% of the patients had high (i.e., >120 RU/ml) FGF23 levels. Median blood phosphate concentration was 0.85 mmol/l (Q1–Q3: 0.74–0.99), and 48% of the patients had low blood phosphate levels (<0.85 mmol/l). The characteristics of the patients according to FGF23 tertiles are presented in Table 2. FGF23 concentration correlated negatively with mGFR (r = -0.41). In addition, FGF23 concentration correlated modestly but significantly with blood phosphate (r = 0.15), PTH (r = 0.15), and calcitriol (r = 0.13) concentrations; body mass index (r = 0.13); systolic blood pressure (r = 0.14); and patient’s age (r = 0.11). It did not correlate significantly with 25-hydroxyvitamin D or ionized blood calcium concentration. In addition to FGF23, mGFR correlated significantly with patient’s age (r = -0.31), calcitriol concentration (r = -0.31), systolic blood pressure (r = -0.27), hemoglobin (r = 0.24), and serum phosphate concentration (r = -0.18) and marginally with ionized blood calcium concentration (r = -0.06). The weak correlation between FGF23 level and serum phosphate concentration suggests that FGF23 was not a main determinant of the hypophosphatemia in our cohort. To assess this possibility, we compared the relation between the 2 main phosphaturic hormones, PTH and FGF23, and serum phosphate levels and maximal reabsorption of phosphate/GFR, which is an indicator of renal phosphate handling (Figure 1). Correlation between serum phosphate concentration or maximal reabsorption of phosphate/GFR was stronger with PTH than with FGF23, suggesting that hypophosphatemia was mainly related to PTH levels rather than to FGF23 at least 12 months after transplantation.
Table 1.
Characteristics of 845 kidney transplant recipients in stable condition in the early posttransplantation period
| Characteristics | (n = 845) |
|---|---|
| Recipients | |
| Age (yr) at inclusion, mean ± SD | 49 (14) |
| Male, n (%) | 492 (58) |
| Initial nephropathy, n (%) | |
| Glomerulonephritis | 139 (16) |
| Cystic kidney disease | 129 (15) |
| Uropathy | 9 (1) |
| Diabetic nephropathy | 57 (7) |
| Vascular nephropathy | 51 (6) |
| Interstitial nephropathy | 23 (3) |
| Congenital anomalies of the kidney and urinary tract | 63 (7) |
| Systemic lupus | 20 (2) |
| Focal and segmental glomerulosclerosis | 35 (4) |
| Genetic glomerular disease | 11 (1) |
| Hemolytic and uremic syndrome | 8 (1) |
| Toxic nephropathy | 6 (1) |
| Others | 22 (2) |
| Unknown | 272 (32) |
| ESRD treatment technique before transplantation, n (%) | |
| Preemptive transplantation | 102 (12) |
| Hemodialysis | 684 (81) |
| Peritoneal dialysis | 33 (4) |
| Peritoneal dialysis and hemodialysis | 17 (2) |
| Functional renal transplant | 8 (1) |
| Duration of dialysis before transplantation (mo), median (Q1–Q3) | 34 (12–70) |
| First transplantation, n (%) | 669 (79) |
| Transplantation vintage, mo, median (Q1–Q3) | 12 (12–13) |
| Donors | |
| Living donor, n (%) | 185 (22) |
| Age (yr), mean ± SD | 53 (16) |
| Extended donor criteria, n (%) | 419/822 (51) |
| Cold ischemia duration (h), median (Q1–Q3) | 18 (9–25) |
| Delayed graft function, n (%) | 249/834 (30) |
| Immunology: HLA mismatch A, B, and DR | |
| < 3 | 347 (41) |
| ≥ 3 | 498 (59) |
| Clinical and biological parameters at first evaluation | |
| BMI, median (Q1–Q3) | 25 (22–28) |
| Systolic blood pressure (mm Hg), median (Q1–Q3) | 132 (120–144) |
| Diastolic blood pressure (mm Hg), median (Q1–Q3) | 75 (69–82) |
| GFR (ml/min per 1.73 m2), median (Q1–Q3) | 54 (42–64) |
| Ionized calcium mmol/l, median (Q1–Q3) | 1.24 (1.20–1.29) |
| Phosphate, mmol/l, median (Q1–Q3) | 0.85 (0.74–0.99) |
| 25-OHD μg/l, median (Q1–Q3) | 23 (15–31) |
| Calcitriol, ng/l, median (Q1–Q3) | 46 (33–63) |
| PTH ng/l, median (Q1–Q3) | 82 (56–136) |
| FGF 23 RU/ml, median (Q1–Q3) | 78 (50–121) |
Categorical variables are presented as numbers (%), and continuous variables are presented as mean (SD) or median (Q1, Q3) as appropriate.
BMI, body mass index; ESRD, end-stage renal disease; FGF, fibroblast growth factor; GFR, glomerular filtration rate; HLA, human leukocyte antigen; 25-OHD, 25-hydroxyvitamin D: PTH, parathyroid hormone; Q1, first quartile; Q3, third quartile.
Table 2.
Characteristics of patients according to FGF23 tertiles
| Characteristics | All patients (n = 845) |
FGF23 tertile 1 (n = 281) |
FGF23 tertile 2 (n = 282) |
FGF23 tertile 3 (n = 282) |
P valuea |
|---|---|---|---|---|---|
| Recipients | |||||
| Age (yr) at inclusion, mean ± SD | 49 ± 14 | 48 ± 13 | 49 ± 14 | 51 ± 14 | 0.02 |
| Gender, male, n (%) | 492 (58) | 165 (59) | 150 (53) | 177 (63) | 0.07 |
| Duration of dialysis before transplantation (mo), median (Q1–Q3) | 34 (12–70) | 32 (11–66) | 36 (13–68) | 36 (13–75) | 0.39 |
| First transplantation | 669 (79) | 222 (79) | 231 (82) | 216 (77) | 0.30 |
| Transplantation vintage (mo), median (Q1–Q3) | 12 (12–13) | 12 (12–13) | 12 (12–13) | 12 (12–14) | 0.51 |
| Donors | |||||
| Living donor | 185 (22) | 75 (27) | 63 (22) | 47 (17) | 0.02 |
| Age (yr), mean ± SD | 53 ± 16 | 51 ± 16 | 51 ± 17 | 57 ± 16 | <0.0001 |
| Cold ischemia duration, h (Q1–Q3) | 18 (9–25) | 17 (3–23) | 18 (4–25) | 19 (13–26) | 0.03 |
| Delayed graft function | 249 (30) | 58/278 (21) | 82/276 (30) | 109/280 (39) | <0.0001 |
| Immunology: HLA mismatch A, B, and DR <3, n (%) | 347 (41) | 128 (46) | 108 (38) | 111 (39) | 0.17 |
| Clinical and biological parameters at first evaluation | |||||
| BMI, median (Q1–Q3) | 25 (22–28) | 24 (22–27) | 24 (21–27) | 25 (23–28) | 0.0004 |
| Systolic blood pressure (mm Hg), median (Q1–Q3) | 132 (120–144) | 130 (119–142) | 130 (118–141) | 136 (123–149) | <0.0001 |
| GFR (ml/min per 1.73 m2), median (Q1–Q3) | 54 (42–64) | 59 (51–69) | 55 (44–64) | 44 (33–56) | <0.0001 |
| Ionized calcium mmol/l, median (Q1–Q3) | 1.24 (1.20–1.29) | 1.24 (1.20–1.29) | 1.24 (1.20–1.29) | 1.24 (1.20–1.30) | 0.98 |
| Phosphate, mmol/l, median (Q1–Q3) | 0.85 (0.74–0.99) | 0.84 (0.74–0.98) | 0.85 (0.73–0.99) | 0.88 (0.75–1.02) | 0.20 |
| Tm phosphate/GFR | 0.62 (0.47–0,79) | 0.65 (0.49–0.80) | 0.65 (0.51–0.81) | 0.57 (0.43–0.76) | 0.0002 |
| 25-OHD μg/l, median (Q1–Q3) | 23 (15–31) | 23 (15–30) | 24 (16–31) | 22 (15–31) | 0.45 |
| Calcitriol, ng/l, median (Q1–Q3) | 46 (33–63) | 46 (34–61) | 50 (35–68) | 44 (31–59) | 0.003 |
| PTH ng/l, median (Q1–Q3) | 82 (56–136) | 70 (51–102) | 80 (57–130) | 106 (65–176) | <0.0001 |
Categorical variables are presented as numbers (%), and continuous variables are presented as mean (SD) or median (Q1, Q3) as appropriate
BMI, body mass index; FGF, fibroblast growth factor; GFR, glomerular filtration rate; 25-OHD, 25-dihydroxyvitamin D; PTH, parathyroid hormone; Q1, first quartile; Q3, third quartile.
P represents tests of significance from one-way analysis of variances, Kruskal-Wallis test, or χ2 test.
Figure 1.
Scatter diagrams illustrating the associations between phosphate, maximal reabsorption of phosphate/glomerular filtration rate (Tm phosphate/GFR), blood phosphate, fibroblast growth factor 23 (FGF23), and parathyroid hormone (PTH).
Association Between FGF23 Concentration and Outcome
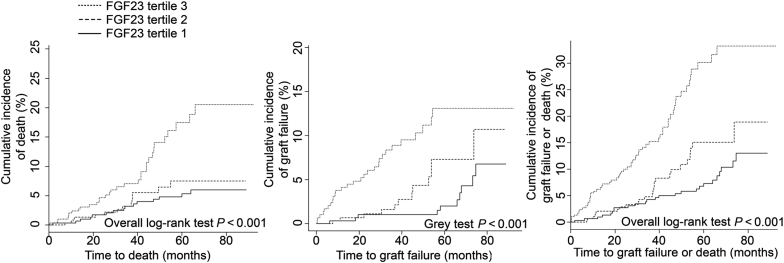
During a median follow-up duration of 71 months, 33 patients were lost to follow-up, 84 lost the allograft, 101 died with a functional allograft, and 4 died with a nonfunctional allograft. The cause of death was uncertain for 24 patients. More than half of the deaths were due to either infections (33 individuals) or neoplasia (24 individuals), and 12 patients died of cardiovascular or cerebrovascular disease. Cumulative incidence plots for graft loss, mortality, or their composite according to FGF23 tertiles are shown in Figure 2. Factors associated with the composite outcome of death or graft failure in univariate analysis are shown in Table 3. The crude hazard ratio (HR) for FGF23, considered as a continuous parameter, was 1.01 (95% confidence interval 1.00–1.01, P = 0.04). Importantly, when adjusted for mGFR, the association was no longer statistically significant (P = 0.07; Table 4). FGF23 did not consistently show a significant association with composite outcome after adjustments for additional predictors of transplantation outcome were made (Table 4). These results were similar when tertiles of FGF23 were considered (Table 4).
Figure 2.
Cumulative incidence of death, graft loss, or the composite outcome across fibroblast growth factor 23 (FGF23) tertiles.
Table 3.
Univariate analysis of characteristics associated with the composite of graft failure or death
| Characteristics | Univariate HR (95% CI) | P value |
|---|---|---|
| Recipients | ||
| -Age at inclusion | 1.03 (1.02–1.04) | <0.0001 |
| -Gender, male | 1.18 (0.87–1.58) | 0.28 |
| -Duration of dialysis before transplantation, mo | 1.003 (1.001–1.005) | 0.0007 |
| -First transplantation | 0.62 (0.45–0.85) | 0.003 |
| -Transplantation vintage, mo | 1.01 (0.99–1.03) | 0.23 |
| Donors | ||
| -Living donor | 0.54 (0.35–0.82) | 0.004 |
| -Age | 1.02 (1.01–1.03) | <0.0001 |
| -Cold ischemia duration, h | 1.03 (1.01–1.04) | <0.0001 |
| -Delayed graft function | 2.18 (1.63–2.92) | <0.0001 |
| - Extended donor criteria | 2.03 (1.50–2.75) | <0.0001 |
| Immunology: HLA mismatch A, B, and DR <3 | 0.96 (0.72–1.29) | 0.78 |
| Clinical and biological parameters at first evaluation | ||
| -BMI | 1.00 (0.97–1.03) | 0.92 |
| -Systolic blood pressure (mm Hg) | 1.02 (1.01–1.03) | <0.0001 |
| -GFR (ml/min per 1.73 m2) | 0.95 (0.94–0.96) | <0.0001 |
| -Ionized blood calcium mmol/l | 2.49 (0.47–13.3) | 0.29 |
| -Phosphate | 3.78 (1.86–7.66) | 0.0002 |
| -25-OHD | 0.98 (0.96–0.99) | 0.0006 |
| -Calcitriol | 0.99 (0.98–0.99) | 0.002 |
| -Log PTH | 3.59 (2.34–5.51) | <0.0001 |
| -FGF23a | 1.01 (1.00–1.01) | 0.04 |
| -FGF23 tertile 3 versus tertile 1 | 2.81 (1.96–4.03) | <0.0001 |
| -FGF23 tertile 2 versus tertile 1 | 1.52 (1.02–2.26) | 0.04 |
BMI, body mass index; CI, confidence interval; FGF, fibroblast growth factor; HLA, human leukocyte antigen; HR, hazard ratio; 25-OHD, 25-dihydroxyvitamin D; PTH, parathyroid hormone.
The hypothesis of proportional hazards was not verified for LogFGF23; therefore FGF23 was used.
Table 4.
Association of fibroblast growth factor 23 with composite outcome after adjustment for mGFR and other variablesa
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| FGF23 adjusted for mGFR | ||
| FGF23 | 1.002 (1.00–1.004) | 0.07 |
| FGF23 adjusted for mGFR and other variablesa | ||
| FGF23 | 1.0002 (0.9999–1.0004) | 0.15 |
| TertileFGF23 (reference = tertile 1) adjusted for mGFR | ||
| FGF23 tertile 2 | 1.15 (0.77–1.72) | 0.50 |
| FGF23 tertile 3 | 1.46 (0.98–2.17) | 0.06 |
| Tertile FGF23 (reference = tertile 1) adjusted for mGFR and other variablesa | ||
| FGF23 tertile 2 | 1.18 (0.77–1.80) | 0.45 |
| FGF23 tertile 3 | 1.33 (0.87–2.04) | 0.19 |
CI, confidence interval; FGF, fibroblast growth factor; mGFR, measured glomerular filtration rate.
Age at inclusion, duration of dialysis, first transplantation, living donor, delayed graft function, systolic blood pressure, phosphate, logparathyroid hormone, logcalcitriol.
These results differed from those of 2 previous studies in which the association between FGF23 and transplantation outcome was explored in transplant recipients in stable condition. Therefore we investigated the origin of this discrepancy.
Influence of GFR Assessment
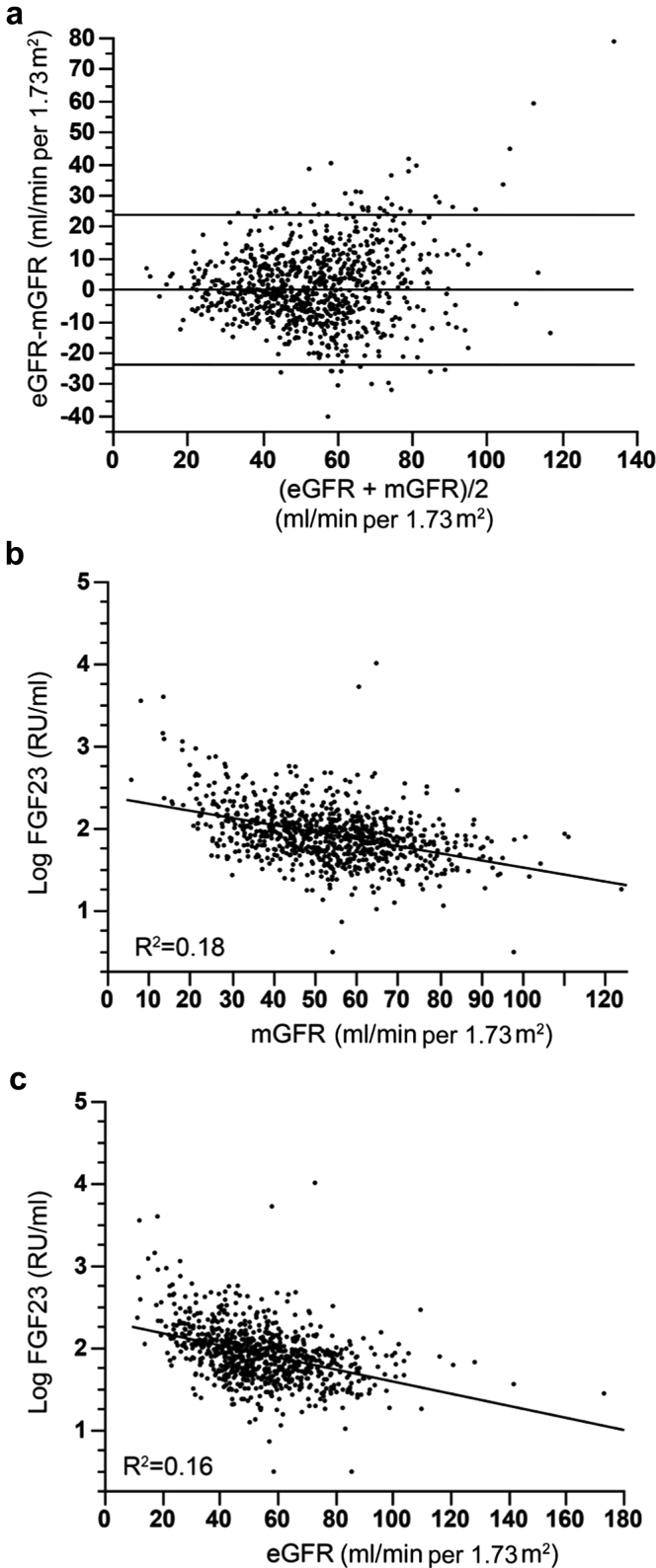
We first examined the impact of the methods used to assess GFR on the association between FGF23 and outcome. Previous studies used creatinine-based estimators of GFR to adjust for kidney function in multivariate analysis. Although eGFR provides a convenient tool for use in making clinical decisions, the MDRD and chronic kidney disease–epidemiology collaboration (CKD-EPI) equations have reduced performance for kidney transplant recipients.17, 19 FG23 plasma level correlates with GFR, and both GFR and FGF23 are associated with composite outcome in univariate analysis. Consequently, we hypothesized that the use of direct measurement of GFR could modify the relation between FGF23 and outcome in kidney transplant recipients. In our cohort, Bland and Altman analysis revealed a limit of agreement of ± 24 ml/min per 1.73m2 between eGFR (calculated according to the simplified MDRD formula) and mGFR (Figure 3). FGF23 level correlated with both mGFR (r = -0.42) and eGFR (r = -0.40). Therefore we conducted a sensitivity analysis using eGFR rather than mGFR to assess whether this modified the association between FGF23 and the outcome. Remarkably, the HR was higher when eGFR was used instead of mGFR, and FGF23 remained significantly associated with the composite outcome after adjustment for eGFR (Table 5). However, after adjustment for multiple factors affecting transplantation outcome, the association with FGF23 was lost. Thus the attenuation of the association between FGF23 and the composite outcome was larger after the adjustment for mGFR compared with eGFR.
Figure 3.
Comparison of measured glomerular filtration rate (mGFR) and estimated glomerular filtration rate (eGFR) according to 3-variable Modification of Diet in Renal Disease (MDRD) formula and their associations with fibroblast growth factor 23 (FGF23). (a) Bland and Altman graphic comparing eGFR and mGFR and scatter diagrams illustrating the association between FGF23 and (b) mGFR or (c) eGFR.
Table 5.
Association of FGF23 with composite outcome after adjustment for eGFR and other variables
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| FGF23 adjusted for eGFR | ||
| FGF23 | 1.0002 (1.000–1.0004) | 0.03 |
| FGF23 adjusted for eGFR and other variablesa | ||
| FGF23 | 1.0002 (1.000–1.0004) | 0.09 |
| Tertile FGF23 (reference = tertile 1) adjusted for eGFR | ||
| FGF23 tertile 2 | 1.31 (0.87–1.96) | 0.19 |
| FGF23 tertile 3 | 1.83 (1.23–2.71) | 0.003 |
| Tertile FGF23 (reference = tertile 1) adjusted for eGFR and other variablesa | ||
| FGF23 tertile 2 | 1.30 (0.85–1.98) | 0.22 |
| FGF23 tertile 3 | 1.51 (0.98–2.33) | 0.06 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; FGF, fibroblast growth factor.
Age at inclusion, duration of dialysis, first transplantation, living donor, delayed graft function, systolic blood pressure, phosphate, logparathyroid hormone, logcalcitriol.
Influence of Follow-up Duration
Another factor that could modify the association between FGF23 level and outcome is the duration of follow-up. In our study, the duration of follow-up was longer than that in previous studies. We used a single FGF23 measurement (as in other studies). Consequently, we could expect that the association between this measurement and the occurrence of events was likely to decrease with time. Both log-transformed FGF23 and FGF23 tertiles were independently associated with the composite outcome in the first 48 months after FGF23 measurement (Table 6).
Table 6.
Association of FGF23 and outcomes in the 48 months after FGF23 measurement after adjustment for mGFR and other variablesa
| Composite outcome within 48 mo | Hazard ratio (95% CI) | P value |
|---|---|---|
| FGF23 adjusted for mGFR | ||
| log FGF23 | 3.36 (2.11–5.33) | <0.0001 |
| FGF23 adjusted for mGFR and other variablesa | ||
| log FGF23 | 3.01 (1.79–5.04) | <0.0001 |
| Tertile FGF23 (reference = tertile 1) adjusted for mGFR | ||
| FGF23 tertile 2 | 1.29 (0.71–2.34) | 0.40 |
| FGF23 tertile 3 | 2.00 (1.14–3.52) | 0.02 |
| Tertile FGF23 (reference = tertile 1) adjusted for mGFR and other variablesa | ||
| FGF23 tertile 2 | 1.38 (0.74–2.60) | 0.31 |
| FGF23 tertile 3 | 1.85 (1.01–3.39) | 0.04 |
| Death within 48 mo | ||
|---|---|---|
| FGF23 adjusted for mGFR | ||
| log FGF23 | 1.62 (0.83–3.13) | 0.15 |
| FGF23 adjusted for mGFR and other variablesa | ||
| log FGF23 | 1.74 (0.85–3.54) | 0.13 |
| Tertile FGF23 (reference = tertile 1) adjusted for mGFR | ||
| FGF23 tertile 2 | 1.00 (0.51–1.96) | 0.99 |
| FGF23 tertile 3 | 1.35 (0.70–2.59) | 0.36 |
| Tertile FGF23 (reference = tertile 1) adjusted for mGFR and other variablesa | ||
| FGF23 tertile 2 | 1.00 (0.50–2.03) | 0.99 |
| FGF23 tertile 3 | 1.37 (0.70–2.71) | 0.36 |
| Graft failure within 48 mo (Fine and Gray model, taking into account competing risk of death) | ||
|---|---|---|
| FGF23 adjusted for mGFR | ||
| log FGF23 | 5.29 (3.07–9.11) | <0.0001 |
| FGF23 adjusted for mGFR and other variablesa | ||
| log FGF23 | 5.74 (2.87–11.49) | <0.0001 |
| Tertile FGF23 (reference = tertile 1) adjusted for mGFR | ||
| FGF23 tertile 2 | 2.75 (0.77–9.75) | 0.12 |
| FGF23 tertile 3 | 4.24 (1.27–14.1) | 0.02 |
| Tertile FGF23 (reference = tertile 1) adjusted for mGFR and other variablesa | ||
| FGF23 tertile 2 | 3.59 (0.78–16.5) | 0.10 |
| FGF23 tertile 3 | 5.02 (1.13–22.3) | 0.03 |
| Graft failure within 48 mo (cause-specific Cox model) | ||
|---|---|---|
| FGF23 adjusted for mGFR | ||
| log FGF23 | 5.46 (2.94–10.2) | <0.0001 |
| FGF23 adjusted for mGFR and other variablesa | ||
| log FGF23 | 5.83 (2.80–12.1) | <0.0001 |
| Tertile FGF23 (reference = tertile 1) adjusted for mGFR | ||
| FGF23 tertile 2 | 2.63 (0.73–9.50) | 0.14 |
| FGF23 tertile 3 | 4.20 (1.21–14.5) | 0.02 |
| Tertile FGF23 (reference = tertile 1) adjusted for mGFR and other variablesa | ||
| FGF23 tertile 2 | 3.30 (0.72–15.2) | 0.12 |
| FGF23 tertile 3 | 4.74 (1.06–21.1) | 0.04 |
CI, confidence interval; FGF, fibroblast growth factor; mGFR, measured glomerular filtration rate.
Age at inclusion, duration of dialysis, first transplantation, living donor, delayed graft function, systolic blood pressure.
These results indicate that FGF23, measured at a median of 12 months after transplantation, is independently associated with the composite outcome in the short-term period (i.e., 48 months) after FGF23 measurement, but not beyond.
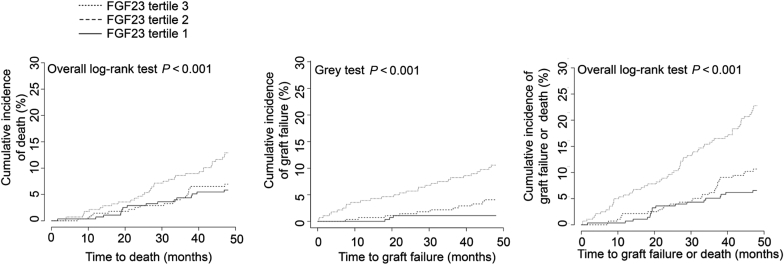
Association Between Graft Failure and Death Separately in the Short-term Period (48 Months)
As secondary analysis, we estimated the association between FGF23 and death or graft failure separately for the short-term period (<48 mo) (Figure 4 and Table 6). In the full model adjusted for mGFR and other variables, FGF23 was independently and strongly associated with graft failure (adjusted HR = 5.74 [95% confidence interval 2.87-11.49]) but not with death (adjusted HR = 1.74 [95% confidence interval 0.85–3.54]).
Figure 4.
Cumulative incidence of death, graft loss, or the composite outcome across fibroblast growth factor 23 (FGF23) tertiles in the 48 months after FGF23 measurement.
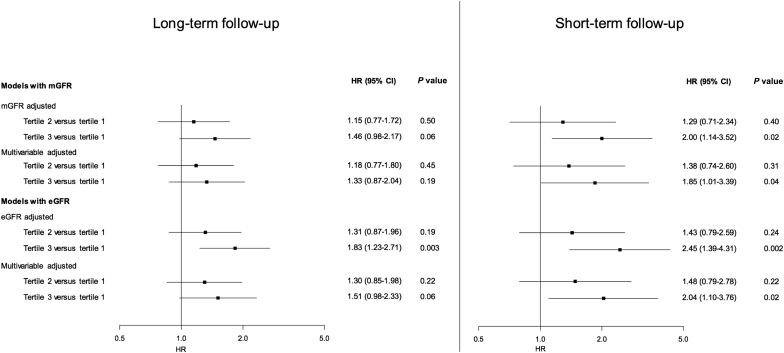
We also conducted a sensitivity analysis using eGFR instead of mGFR to assess whether this could have an influence on our results. The use of eGFR yielded consistent results with analyses based on mGFR for composite outcome or graft loss, although the HRs were larger with mGFR than with eGFR. The association between log-transformed FGF23 and death within 48 months became statistically significant when eGFR was used instead of mGFR (compare Table 6 and Table 7). The HR for the association of FGF23 tertiles with the composite outcome according to follow-up duration and the method used to assess GFR are summarized in Figure 5.
Table 7.
Association of FGF23 and outcomes in the 48 months following FGF23 after adjustment for eGFR and other variablesa
| Composite outcome within 48 mo | Hazard ratios (95% CI) | P value |
|---|---|---|
| FGF23 adjusted for eGFR | ||
| log FGF23 | 4.01 (2.62–6.14) | <0.0001 |
| FGF23 adjusted for eGFR and other variablesa | ||
| log FGF23 | 3.26 (1.98–5.40) | <0.0001 |
| Tertile FGF23 (reference = tertile 1) adjusted for eGFR | ||
| FGF23 tertile 2 | 1.43 (0.79–2.59) | 0.24 |
| FGF23 tertile 3 | 2.45 (1.39–4.31) | 0.002 |
| Tertile FGF23 (reference = tertile 1) adjusted for eGFR and other variablesa | ||
| FGF23 tertile 2 | 1.48 (0.79–2.78) | 0.22 |
| FGF23 tertile 3 | 2.04 (1.10–3.76) | 0.02 |
| Death within 48 mo | ||
|---|---|---|
| FGF23 adjusted for eGFR | ||
| log FGF23 | 2.32 (1.27–4.24) | 0.006 |
| FGF23 adjusted for eGFR and other variablesa | ||
| log FGF23 | 2.04 (1.03–4.03) | 0.04 |
| Tertile FGF23 (reference = tertile 1) adjusted for eGFR | ||
| FGF23 tertile 2 | 1.12 (0.57–2.19) | 0.74 |
| FGF23 tertile 3 | 1.80 (0.95–3.45) | 0.07 |
| Tertile FGF23 (reference = tertile 1) adjusted for eGFR and other variablesa | ||
| FGF23 tertile 2 | 1.05 (0.52–2.12) | 0.90 |
| FGF23 tertile 3 | 1.57 (0.79–3.10) | 0.19 |
| Graft failure within 48 mo (Fine and Gray model, taking into account competing risk of death) | ||
|---|---|---|
| FGF23 adjusted for eGFR | ||
| log FGF23 | 5.83 (3.34–10.16) | <0.0001 |
| FGF23 adjusted for eGFR and other variablesa | ||
| log FGF23 | 6.22 (3.05–12.7) | <0.0001 |
| Tertile FGF23 (reference = tertile 1) adjusted for eGFR | ||
| FGF23 tertile 2 | 2.79 (0.77–10.11) | 0.12 |
| FGF23 tertile 3 | 4.16 (1.13–15.3) | 0.03 |
| Tertile FGF23 (reference = tertile 1) adjusted for eGFR and other variablesa | ||
| FGF23 tertile 2 | 3.67 (0.78–17.1) | 0.10 |
| FGF23 tertile 3 | 4.84 (0.99–23.5) | 0.05 |
| Graft failure within 48 mo (cause-specific Cox model) | ||
|---|---|---|
| FGF23 adjusted for eGFR | ||
| log FGF23 | 6.08 (3.3–11.2) | <0.0001 |
| FGF23 adjusted for eGFR and other variablesa | ||
| log FGF23 | 6.38 (3.12–13.03) | <0.0001 |
| Tertile FGF23 (reference = tertile 1) adjusted for eGFR | ||
| FGF23 tertile 2 | 2.71 (0.75–9.82) | 0.13 |
| FGF23 tertile 3 | 4.21 (1.20–14.7) | 0.02 |
| Tertile FGF23 (reference = tertile 1) adjusted for eGFR and other variablesa | ||
| FGF23 tertile 2 | 3.51 (0.77–16.1) | 0.11 |
| FGF23 tertile 3 | 4.75 (1.06–21.4) | 0.04 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; FGF, fibroblast growth factor.
Age at inclusion, time on dialysis, first transplantation, living donor, delayed graft function, and systolic blood pressure.
Figure 5.
Comparison of the hazard ratio (HR) for the association of FGF23 tertiles with the composite outcome according to follow-up duration and the method used to assess glomerular filtration rate (GFR). CI, confidence interval.
Association Between FGF23 and Cardiac Hypertrophy
The heart has emerged as an important target organ for FGF23 signaling in CKD. Indeed, FGF23 concentration correlates with left ventricular hypertrophy and cardiac remodeling in the population with CKD, and exposure to high FGF23 concentrations induces cardiomyocyte hypertrophy in vitro and increased left ventricular mass in rodents in vivo.10 Whether FGF23 levels are associated with left ventricular mass after transplantation remains unknown. To investigate this possibility, we analyzed all the echocardiographic findings obtained at our institution. Two hundred twenty-seven patients in our cohort had echocardiography performed within 3 months of FGF23 and mGFR assessments. Echocardiography was performed within 7 days after or before FGF23 measurement in 75% of the patients. We found no correlation between FGF23 and indexed left ventricular mass (Table 8). In contrast, patient age, systolic blood pressure, duration of dialysis, and mGFR were associated with indexed left ventricular mass. None of the studied parameters was consistently associated with left ventricle ejection fraction.
Table 8.
Correlations of left ventricular mass and left ventricular ejection fraction with demographic, clinical, an biological parameters in the 277 kidney transplant recipients with available echocardiography
| LVM (mg/1.73m2) | LVEF (%) | |
|---|---|---|
| Median (Q1–Q3) | 124 (105–148) | 67 (61–72) |
| Mean ± SD | 129 ± 36 | 67 ± 8 |
| Parameter | ρa | P | ρa | P |
|---|---|---|---|---|
| Age at exploration | 0.23 | 0.0001 | -0.09 | 0.14 |
| Duration of dialysis | 0.25 | <0.0001 | -0.12 | 0.04 |
| Systolic blood pressure | 0.18 | 0.002 | -0.10 | 0.11 |
| mGFR | -0.20 | 0.0009 | 0.10 | 0.10 |
| Hemoglobin | -0.03 | 0.66 | -0.03 | 0.65 |
| 1-25-OHD | -0.09 | 0.15 | 0.006 | 0.92 |
| 25-OHD | -0.10 | 0.09 | -0.01 | 0.83 |
| FGF23 | 0.08 | 0.20 | -0.04 | 0.51 |
| PTH | 0.13 | 0.03 | 0.08 | 0.16 |
FGF, fibroblast growth factor; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; mGFR, measured glomerular filtration rate; 1-25-OHD, 1,25-dihydroxyvitamin D; 25-OHD, 25-dihydroxyvitamin D; PTH, parathyroid hormone; Q1, quartile 1; Q3, quartile 3.
P values < 0.05 are in boldface type.
Spearman’s correlation coefficient.
Discussion
In this large, prospective single-center cohort of recent kidney transplant recipients in stable condition with a prolonged follow-up, we analyzed the association between FGF23 and graft failure or death. The first striking finding is that FGF23 plasma level was not associated with outcomes independently of mGFR. These results contrast with those of 2 cross-sectional studies that explored the association between FGF23 and outcomes in kidney transplant recipients in stable condition.14, 15 Several factors that are not mutually exclusive may contribute to an explanation for this discrepancy. Both Wolf et al. and Baia et al. used creatinine-based estimators of GFR.14, 15 However, both MDRD and CKD-EPI equations have reduced performance for kidney transplant recipients.17, 19 Several factors—including variation in muscle mass and metabolism, variation in creatinine tubular secretion owing to drugs,20 weight,21 and genetic variation in the creatinine tubular transporter SLC22A222—likely contribute to this reduced performance. We observed that using mGFR, rather than eGFR, as an adjustment for kidney function reduced the apparent effect of FGF23 on the composite outcome. Because FGF23 showed a better correlation with mGFR than eGFR, it is likely that the use of mGFR allows a better adjustment for kidney function in multivariate analysis, resulting in a reduction of the apparent weight of FGF23. The results of our study exemplify the fact that the use of a creatinine-based equation to adjust for kidney function in multivariate analysis may lead to the erroneous conclusion that a variable affects outcome independently of GFR, especially in populations for whom these equations have reduced performance. This observation represents a serious issue for studies aiming to identify factors independently associated with outcome in renal transplantation. During the period of the study, creatinine measurements were not calibrated on isotope dilution mass spectrometry standards at our institution. Therefore we used the MDRD formula and not the CKD-EPI equation, which requires isotope dilution mass spectrometry calibration. The CKD-EPI equation provides a better estimation of GFR than the MDRD formula for individuals with high GFR who have not undergone renal transplantation, but it remains unclear whether it offers an advantage for kidney transplant recipients.17 Whether the CKD-EPI equation or other new creatinine and/or cystatin-based formulas estimate mGFR with sufficient accuracy to prevent an erroneous conclusion in multivariate analysis remains to be determined.
Our study also points out an important impact of follow-up duration on the apparent association between FGF23 and outcome. We observed that the association between FGF23 and outcome was only significant in the first 4 years after FGF23 measurement. This loss of association with long-term follow-up is not surprising. Nakano et al. reported that pre-dialysis FGF23 levels predicted cardiovascular events before dialysis was started but not in the postdialysis period.23 However, in this study the initiation of dialysis could have altered the relation between FGF23 and outcome. In our study, there were no such modifications in patient treatments. We did not include repeated FGF23 measurements in our study; consequently, we could not determine whether repeated measurements of FGF23 would be better associated with outcome than a single assessment of FGF23. Our results underline important methodological issues regarding the evaluation of the relationship between GFR-linked variables, such as FGF23, and outcomes in kidney transplant recipients in the early posttransplantation period. These critical elements should be taken into account when future studies on this topic are designed.
Another important conclusion of our study is that, contrary to the observation made in patients with stable CKD, FGF23 is not an independent risk factor for mortality in the first 4 years after kidney transplantation. The early posttransplantation period is associated with several modifications in mineral metabolism, including urinary phosphate leakage and a lower blood phosphate level.24 Interestingly, the association between FGF23 and death or cardiovascular events is reduced in patients with a high renal fraction of phosphate excretion and a lower blood phosphate level who have not undergone renal transplantation.25 Also, it is not known whether the FGF23 concentration in patients who have hypophosphatemic rickets caused by FGF23 overproduction is associated with increased risk of cardiovascular death.26 The association between FGF23 and cardiovascular disease and death has been demonstrated mainly in cohorts of patients who mostly presented with normal or high blood phosphate levels. In line with these observations, our results show that in a large cohort of patients with a high incidence of renal hypophosphatemia, FGF23 is not independently associated with death and supports the theory that renal phosphate leakage could mitigate the potential deleterious effects of FGF23. Remarkably, deaths of cardiovascular origin were relatively rare in our cohort compared with the incidence reported in previous studies of FGF23 in renal transplant recipients. This may simply reflect the specific recruitment of patients at our institution: our cohort included relatively few patients with diabetes and hypertension as a cause of end-stage renal disease. Alternatively, one could speculate that renal phosphate leakage (or another factor) mitigates the effects of FGF23 on cardiovascular health, thereby reducing the incidence of cardiovascular events. The potential mechanisms underlying the apparently reduced cardiovascular risk associated with FGF23 in patients with renal phosphate leakage remain unclear. It has been proposed that renal phosphate leakage marks patients with a preserved expression of the FGF23 co-receptor Klotho in the kidney.27 Klotho has been shown to exert cardiovascular protective effects at least in rodent models.28 Thus renal phosphate leakage in patients with high FGF23 concentrations may indicate higher αKlotho expression with a positive impact on cardiovascular health. However, in our cohort, phosphate renal leakage appeared to be determined primarily by PTH, which exerts its phosphaturic effect independently of αKlotho.29 Concomitant measurement of FGF23 and αKlotho in large cohorts of patients with or without renal phosphate leakage will help to disentangle the complex interplay between FGF23 and αKlotho renal phosphate handling in patients who have undergone kidney transplantation.
FGF23 is believed to play a causal role in left ventricle hypertrophy in patients with CKD, and this effect has been proposed to be responsible for its association with death in this population. To determine whether this association was present in the early posttransplantation period, we analyzed a subgroup of patients for whom echocardiographic findings were available. Remarkably, at first glance in studies performed in the population with CKD, we did not find any association between FGF23 levels and left ventricular mass. Several potential explanations may be proposed. First, FGF23 has been shown to mediate cardiomyocyte hypertrophy in a calcineurin-dependent manner in vitro.10, 11 Most of the patients in this study received an anticalcineurin-based immunosuppressive regimen, which may have antagonized the impact of FGF23 on left ventricular mass. Alternatively, because FGF23 levels are drastically reduced after kidney transplantation and because most of the patients were enrolled only 1 year after transplantation, the exposure time to posttransplantation FGF23 levels may be too short to have an impact on left ventricular mass. Indeed, at this time point, left ventricular mass seems to be drastically imprinted by the pretransplantation period, as demonstrated by its robust association with the duration of dialysis. Also, we cannot exclude that longer follow-up would unveil an association between FGF23 and left ventricular mass. The fact that this association was absent at the time of inclusion, combined with the absence of an independent association between FGF23 and death, argues against a major impact of early posttransplantation FGF23 levels on cardiac health in the first years after renal transplantation.
The emergence of multiple deleterious pathophysiologic roles for FGF23 in CKD has received considerable attention recently. Treatments aimed at neutralizing deleterious FGF23 signaling are emerging.30 However, antagonizing FGF23 is associated with deleterious effects in rodent models of CKD.7 It is therefore of primary importance to determine in which population FGF23 likely represents a real risk factor. In this context, our study highlights methodological issues regarding the significance of FGF23 in renal transplantation.
Disclosure
DP received honoraria from AMGEN for lectures. All the other authors declared no competing interests.
References
- 1.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.USRDS . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. US Renal Data System, USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. [Google Scholar]
- 3.Goetz R., Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14:166–180. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olauson H., Vervloet M.G., Cozzolino M. New insights into the FGF23-Klotho axis. Semin Nephrol. 2014;34:586–597. doi: 10.1016/j.semnephrol.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Olauson H., Lindberg K., Amin R. Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLoS Genet. 2013;9:e1003975. doi: 10.1371/journal.pgen.1003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isakova T., Wahl P., Vargas G.S. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shalhoub V., Shatzen E.M., Ward S.C. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122:2543–2553. doi: 10.1172/JCI61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutiérrez O.M., Mannstadt M., Isakova T. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isakova T., Xie H., Yang W. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faul C., Amaral A.P., Oskouei B. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grabner A., Amaral A.P., Schramm K. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22:1020–1032. doi: 10.1016/j.cmet.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chonchol M., Greene T., Zhang Y. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO Study. J Am Soc Nephrol. 2016;27:227–237. doi: 10.1681/ASN.2014101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossaint J., Oehmichen J., Van Aken H. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin. Invest. 2016;126:962–974. doi: 10.1172/JCI83470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf M., Molnar M.Z., Amaral A.P. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22:956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baia L.C., Humalda J.K., Vervloet M.G. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin J Am Soc Nephrol. 2013;8:1968–1978. doi: 10.2215/CJN.01880213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courbebaisse M., Thervet E., Souberbielle J.C. Effects of vitamin D supplementation on the calcium-phosphate balance in renal transplant patients. Kidney Int. 2009;75:646–651. doi: 10.1038/ki.2008.549. [DOI] [PubMed] [Google Scholar]
- 17.Masson I., Flamant M., Maillard N. MDRD versus CKD-EPI equation to estimate glomerular filtration rate in kidney transplant recipients. Transplantation. 2013;95:1211–1217. doi: 10.1097/TP.0b013e318288caa6. [DOI] [PubMed] [Google Scholar]
- 18.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. Available at: http://www.R-project.org/.
- 19.Kukla A., El-Shahawi Y., Leister E. GFR-estimating models in kidney transplant recipients on a steroid-free regimen. Nephrol Dial Transpl. 2010;25:1653–1661. doi: 10.1093/ndt/gfp668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen N.V., Ladefoged S.D., Feldt-Rasmussen B. The effects of cimetidine on creatinine excretion, glomerular filtration rate and tubular function in renal transplant recipients. Scand J Clin Lab Invest. 1989;49:155–159. doi: 10.3109/00365518909105415. [DOI] [PubMed] [Google Scholar]
- 21.Sinkeler S.J., Visser F.W., Krikken J.A. Higher body mass index is associated with higher fractional creatinine excretion in healthy subjects. Nephrol Dial Transplant. 2011;26:3181–3188. doi: 10.1093/ndt/gfq850. [DOI] [PubMed] [Google Scholar]
- 22.Reznichenko A., Sinkeler S.J., Snieder H. SLC22A2 is associated with tubular creatinine secretion and bias of estimated GFR in renal transplantation. Physiol Genomics. 2013;45:201–209. doi: 10.1152/physiolgenomics.00087.2012. [DOI] [PubMed] [Google Scholar]
- 23.Nakano C., Hamano T., Fujii N. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone. 2012;50:1266–1274. doi: 10.1016/j.bone.2012.02.634. [DOI] [PubMed] [Google Scholar]
- 24.Baia L.C., Heilberg I.P., Navis G. Phosphate and FGF-23 homeostasis after kidney transplantation. Nat Rev Nephrol. 2015;11:656–666. doi: 10.1038/nrneph.2015.153. [DOI] [PubMed] [Google Scholar]
- 25.Dominguez J.R., Shlipak M.G., Whooley M.A. Fractional excretion of phosphorus modifies the association between fibroblast growth factor-23 and outcomes. J Am Soc Nephrol. 2013;24:647–654. doi: 10.1681/ASN.2012090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White K.E., Hum J.M., Econs M.J. Hypophosphatemic rickets: revealing novel control points for phosphate homeostasis. Curr Osteoporos Rep. 2014;12:252–262. doi: 10.1007/s11914-014-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bech A.P., Bouma-de Krijger A., van Zuilen A.D. Impact of fractional phosphate excretion on the relation of FGF23 with outcome in CKD patients. J Nephrol. 2015;28:477–484. doi: 10.1007/s40620-015-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie J., Yoon J., An S. Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol. 2015;26:1150–1160. doi: 10.1681/ASN.2014040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan Q., Sato T., Densmore M. FGF-23/Klotho signaling is not essential for the phosphaturic and anabolic functions of PTH. J Bone Miner Res. 2011;26:2026–2035. doi: 10.1002/jbmr.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukumoto S. Anti-fibroblast growth factor 23 antibody therapy. Curr Opin Nephrol Hypertens. 2014;23:346–351. doi: 10.1097/01.mnh.0000447012.98357.da. [DOI] [PubMed] [Google Scholar]