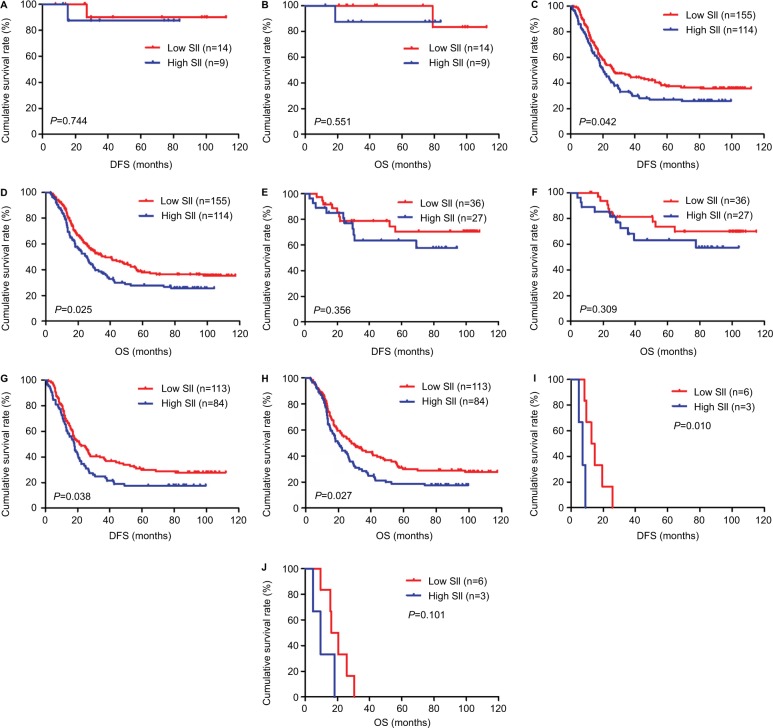
Figure 3.
DFS and OS for the SII of patients with gastric cancer in pathologic stage.
Notes: (A) Kaplan–Meier analysis of DFS for the SII of patients with gastric cancer in early stage gastric cancer. (B) Kaplan–Meier analysis of OS for the SII of patients with gastric cancer in early stage gastric cancer. (C) Kaplan–Meier analysis of DFS for the SII of patients with gastric cancer in advanced stage gastric cancer. (D) Kaplan–Meier analysis of OS for the SII of patients with gastric cancer in advanced stage gastric cancer. (E) Kaplan–Meier analysis of DFS for the SII of patients with gastric cancer in pathologic II stage. (F) Kaplan–Meier analysis of OS for the SII of patients with gastric cancer in pathologic II stage. (G) Kaplan–Meier analysis of DFS for the SII of patients with gastric cancer in pathologic III stage. (H) Kaplan–Meier analysis of OS for the SII of patients with gastric cancer in pathologic III stage. (I) Kaplan–Meier analysis of DFS for the SII of patients with gastric cancer in pathologic IV stage. (J) Kaplan–Meier analysis of OS for the SII of patients with gastric cancer in pathologic IV stage. SII is a novel systemic immune–inflammation index (SII=N×P/L), which is based on neutrophil (N), platelet (P) and lymphocyte (L) counts.
Abbreviations: DFS, disease-free survival; OS, overall survival.