Abstract
Objectives
Bacterial meningitis remains an important cause of morbidity and mortality worldwide. Its epidemiological characteristics, however, are changing due to new vaccines and secular trends. Conjugate vaccines against Haemophilus influenzae type b and Streptococcus pneumoniae (10-valent) were introduced in 1986 and 2010 in Finland. We assessed the disease burden and long-term trends of five common causes of bacterial meningitis in a population-based observational study.
Methods
A case was defined as isolation of S. pneumoniae, Neisseria meningitidis, Streptococcus agalactiae, Listeria monocytogenes or H. influenzae from cerebrospinal fluid and reported to national, population-based laboratory surveillance system during 1995–2014. We evaluated changes in incidence rates (Poisson or negative binomial regression), case fatality proportions (χ2) and age distribution of cases (Wilcoxon rank-sum).
Results
During 1995–2014, S. pneumoniae and N. meningitidis accounted for 78% of the total 1361 reported bacterial meningitis cases. H. influenzae accounted for 4% of cases (92% of isolates were non-type b). During the study period, the overall rate of bacterial meningitis per 1 00 000 person-years decreased from 1.88 cases in 1995 to 0.70 cases in 2014 (4% annual decline (95% CI 3% to 5%). This was primarily due to a 9% annual reduction in rates of N. meningitidis (95% CI 7% to 10%) and 2% decrease in S. pneumoniae (95% CI 1% to 4%). The median age of cases increased from 31 years in 1995–2004 to 43 years in 2005–2014 (p=0.0004). Overall case fatality proportion (10%) did not change from 2004 to 2009 to 2010–2014.
Conclusions
Substantial decreases in bacterial meningitis were associated with infant conjugate vaccination against pneumococcal meningitis and secular trend in meningococcal meningitis in the absence of vaccination programme. Ongoing epidemiological surveillance is needed to identify trends, evaluate serotype distribution, assess vaccine impact and develop future vaccination strategies.
Keywords: Epidemiology, meningitis
Strengths and limitations of this study.
This study describes the epidemiological characteristics of >1300 cases of bacterial meningitis reported to national surveillance during 20 years in Finland.
The study provides clinically important information on the changing distribution of pathogens and age of cases.
The study documents the sustained population impact of infant conjugate vaccination against Haemophilus influenzae type b; and introduction of 10-valent pneumococcal conjugate vaccination on reducing the burden of bacterial meningitis, as well as decline in meningococcal meningitis due to secular trend. As the data were from laboratory-based surveillance system, clinical information such as severity or treatment was not available.
Incidence rate of bacterial meningitis may be underestimated since cases diagnosed by PCR or antigen detection and culture-negative meningitis cases diagnosed based on clinical symptoms and findings were not included in the dataset.
Introduction
Despite the availability of vaccines, antibiotics and advances in intensive care, bacterial meningitis remains an important cause of morbidity and mortality worldwide. Persistent neurological sequelae including hearing loss, neuropsychological impairment or seizures are reported in 10%–30% of survivors.1 The case fatality proportion (CFP) ranges from 5% to 30% for different bacteria.2 3
Globally, Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae are the most important causes of bacterial meningitis, particularly in young children.4 5 Among neonates, the most common cause of bacterial meningitis is S. agalactiae,2 6 while Listeria monocytogenes is important in newborns and elderly persons with comorbidities.7 However, the leading organisms causing bacterial meningitis vary by age of the patient, time and geographical location.5 As the choice of empirical antimicrobial treatment for bacterial meningitis should be based on local epidemiology, patient’s age, presence of risk factors and regional resistance patterns,8–10 population-based surveillance data are important to help in formulating clinical guidelines.
The introduction of effective protein conjugate vaccines against H. influenzae type b (Hib), S. pneumoniae and N. meningitidis has changed the epidemiology of bacterial meningitis in many countries.11 12 In Finland, universal vaccination against Hib since 1986 resulted in rapid elimination of the disease13 and introduction of the 10-valent pneumococcal conjugate vaccine (PCV10) in September 2010 has resulted in substantial reduction in vaccine-type invasive disease.14 15 Meningococcal conjugate vaccines (MCVs) have not been introduced into Finnish National Vaccination Programme (NVP). However, meningococcal polysaccharide vaccine has been offered to military conscripts since 1982.
To provide information for developing future prevention strategies and to help in formulating clinical guidelines, we conducted a population-based observational study to determine the contribution of specific pathogens to the total bacterial meningitis disease burden and to assess long-term trends in the incidence of common aetiologies in Finland during 1995–2014.
Materials and methods
Data sources
Since 1995, all clinical microbiology laboratories in Finland have had legal obligation to report microbial isolations from blood and/or cerebrospinal fluid (CSF) to the National Infectious Diseases Register (NIDR)—a population-based, electronic laboratory surveillance system maintained by the National Institute for Health and Welfare (THL). Routinely collected information include the microbe, specimen date, date of birth, sex, place of residence and unique Personal Identity Code (PIC). For blood or CSF findings concerning S. pneumoniae, S. agalactiae, N. meningitidis, L. monocytogenes or H. influenzae, multiple notifications with the same PIC and microbe are merged into one case if they occurred within 3 months of the first notification. Since 2004, information on vital status after episode is routinely obtained from the Population Information System. All clinical microbiology laboratories also submit isolates from reported cases to THL reference laboratories for species verification and characterisation of the isolates including serotyping or serogrouping. Since 2004, serotyping results are linked to NIDR notifications by using the PIC. Antimicrobial susceptibility data were not available.
Case definitions
We defined a case of bacterial meningitis as isolation of S. pneumoniae, S. agalactiae, N. meningitidis, L. monocytogenes or H. influenzae from CSF and notified to NIDR from 1995 through 2014.
For cases reported during 2004–2014, we calculated the pathogen-specific 30-day CFP as number of cases resulting in death within 30 days from the first positive CSF culture, divided by all cases.
We calculated the proportions of S. pneumoniae, N. meningitidis and H. influenzae cases due to vaccine-preventable serotypes/serogroups during 2004–2014. Serotypes covered in PCV10 are the following: 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F; the 13-valent PCV13 adds serotypes 3, 6A and 19A. Vaccine-preventable meningococcal serogroups include those in the quadrivalent MCV (MCV-4, A, C, W and Y) and serogroup B isolates targeted by novel protein-based vaccines (MenB). For H. influenzae, type b was considered vaccine preventable.
Statistical analysis
By using data from the Population Information System as denominators, we calculated pathogen-specific and age-specific annual incidence rates. Poisson regression was used to test for log-linear trend in rates of bacterial meningitis during 1995–2014. Incidence rate ratios (IRRs), their 95% CI and p values for yearly changes were calculated using time (year) as a continuous explanatory variable in the Poisson model. When appropriate, we used negative binomial regression to correct for overdispersion of data. To compare age distribution of cases across years, we used Wilcoxon rank-sum test. To assess changes in CFP, we used χ2 analyses; p value <0.05 was considered statistically significant. All analyses were done with STATA version 13 and Microsoft Excel 2013.
Ethical considerations
Data used in the analysis were collected as a part of national routine surveillance which falls under the existing mandate of THL. No formal Institutional Review Board review was required for this study. Personal identifiers were removed after linkage with vital status data.
Results
Overall incidence rates of bacterial meningitis
From 1995 to 2014, 1361 cases of bacterial meningitis caused by S. pneumoniae, N. meningitidis, S. agalactiae, L. monocytogenes or H. influenzae were reported (mean incidence rate, 1.29 cases/100 000 person-years, table 1). S. pneumoniae and N. meningitidis were the most common aetiologies accounting for 78% (1061/1361) of cases. The median age of cases increased from 31 years in 1995–2004 to 43 years in 2005–2014 (p=0.0004). Rates were higher in men than women (1.52 vs 1.07 cases/100 000 person-years; IRR 1.4, 95% CI 1.3 to 1.6)).
Table 1.
Number of cases (n), incidence rates per 100 000 person-years (IR) and mean annual relative change in incidence of bacterial meningitis, according to age group (years), Finland, 1995–2014
| 1995–1999 | 2000–2004 | 2005–2009 | 2010–2014 | 1995–2014 | 1995–2014 | |||||||
| n | IR | n | IR | n | IR | n | IR | n | IR | % change* | 95% CI | |
| Streptococcus pneumoniae | ||||||||||||
| <2 | 26 | 4.32 | 25 | 4.43 | 18 | 3.05 | 14 | 2.33 | 83 | 3.52 | −4 | −7 to 0 |
| 2–4 | 6 | 0.63 | 3 | 0.35 | 6 | 0.69 | 3 | 0.33 | 18 | 0.50 | −1 | −8 to 7 |
| 5–17 | 13 | 0.31 | 11 | 0.26 | 7 | 0.17 | 2 | 0.05 | 33 | 0.20 | −7 | −13 to 1 |
| 18–49 | 59 | 0.50 | 54 | 0.48 | 35 | 0.32 | 30 | 0.27 | 178 | 0.40 | −4 | −6 to 1 |
| 50–64 | 41 | 0.91 | 52 | 1.00 | 56 | 0.99 | 37 | 0.65 | 186 | 0.88 | −2 | −4 to 1 |
| ≥65 | 25 | 0.67 | 23 | 0.57 | 39 | 0.89 | 26 | 0.51 | 113 | 0.66 | −1 | −4 to 2 |
| All age groups | 170 | 0.55 | 168 | 0.65 | 161 | 0.61 | 112 | 0.41 | 611 | 0.58 | −2 | −4 to 1 |
| Neisseria meningitidis | ||||||||||||
| <2 | 23 | 3.83 | 23 | 4.07 | 8 | 1.36 | 14 | 2.33 | 68 | 2.89 | −4 | −8 to 0 |
| 2–4 | 19 | 1.98 | 11 | 1.27 | 12 | 1.38 | 6 | 0.66 | 48 | 1.33 | −6 | −10 to −1 |
| 5–17 | 37 | 0.88 | 16 | 0.38 | 24 | 0.60 | 7 | 0.18 | 84 | 0.52 | −8 | −14 to −3 |
| 18–49 | 93 | 0.79 | 46 | 0.41 | 42 | 0.38 | 14 | 0.13 | 195 | 0.43 | −10 | −13 to −8 |
| 50–64 | 15 | 0.33 | 15 | 0.29 | 7 | 0.12 | 2 | 0.04 | 39 | 0.18 | −12 | −17 to −6 |
| ≥65 | 6 | 0.16 | 3 | 0.07 | 4 | 0.09 | 3 | 0.06 | 16 | 0.09 | −7 | −14 to 2 |
| All age groups | 193 | 0.62 | 114 | 0.44 | 97 | 0.37 | 46 | 0.17 | 450 | 0.43 | −9 | −10 to −7 |
| Haemophilus influenzae | ||||||||||||
| <2 | 4 | 0.67 | 3 | 0.53 | 2 | 0.34 | 1 | 0.17 | 10 | 0.42 | −7 | −17 to 4 |
| 2–4 | 0 | 0.00 | 3 | 0.35 | 0 | 0.00 | 0 | 0.00 | 3 | 0.08 | NA | NA |
| 5–17 | 5 | 0.12 | 4 | 0.10 | 0 | 0.00 | 2 | 0.05 | 11 | 0.07 | −8 | −17 to 3 |
| 18–49 | 4 | 0.03 | 2 | 0.02 | 2 | 0.02 | 6 | 0.05 | 14 | 0.03 | 5 | −5 to 15 |
| 50–64 | 3 | 0.07 | 3 | 0.06 | 2 | 0.04 | 2 | 0.04 | 10 | 0.05 | −3 | −13 to 8 |
| ≥65 | 2 | 0.05 | 0 | 0.00 | 7 | 0.16 | 1 | 0.02 | 10 | 0.06 | 1 | −9 to 12 |
| All age groups | 18 | 0.06 | 15 | 0.06 | 13 | 0.05 | 12 | 0.04 | 58 | 0.06 | −2 | −7 to 2 |
| S. agalactiae | ||||||||||||
| <2 | 25 | 4.16 | 24 | 4.25 | 32 | 5.43 | 25 | 4.16 | 106 | 4.50 | 0 | −3 to 5 |
| 2–4 | 1 | 0.10 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.03 | NA | NA |
| 5–17 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 | NA | NA |
| 18–49 | 2 | 0.02 | 1 | 0.01 | 1 | 0.01 | 2 | 0.02 | 6 | 0.01 | 1 | −12 to 16 |
| 50–64 | 4 | 0.09 | 2 | 0.04 | 7 | 0.12 | 3 | 0.05 | 16 | 0.08 | 1 | −9 to 8 |
| ≥65 | 0 | 0.00 | 7 | 0.17 | 1 | 0.02 | 3 | 0.06 | 11 | 0.06 | −2 | −11 to 9 |
| All age groups | 32 | 0.10 | 35 | 0.13 | 41 | 0.15 | 33 | 0.12 | 141 | 0.13 | 0 | −3 to 3 |
| Listeria monocytogenes | ||||||||||||
| <2 | 1 | 0.17 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.04 | NA | NA |
| 2–4 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | NA | NA |
| 5–17 | 1 | 0.02 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 | NA | NA |
| 18–49 | 9 | 0.08 | 3 | 0.03 | 0 | 0.00 | 3 | 0.03 | 15 | 0.03 | −11 | −19 to −3 |
| 50–64 | 10 | 0.22 | 3 | 0.06 | 6 | 0.11 | 4 | 0.07 | 23 | 0.11 | −6 | −13 to 1 |
| ≥65 | 14 | 0.37 | 13 | 0.32 | 13 | 0.30 | 21 | 0.42 | 61 | 0.35 | 0 | −4 to 4 |
| All age groups | 35 | 0.11 | 19 | 0.07 | 19 | 0.07 | 28 | 0.10 | 101 | 0.10 | −2 | −5 to 1 |
| Total bacterial meningitis | ||||||||||||
| <2 | 79 | 13.14 | 75 | 13.28 | 60 | 10.18 | 54 | 8.99 | 268 | 11.38 | −2 | −4 to 1 |
| 2–4 | 26 | 2.71 | 17 | 1.97 | 18 | 2.07 | 9 | 0.98 | 70 | 1.94 | −5 | −10 to 0 |
| 5–17 | 56 | 1.33 | 32 | 0.77 | 31 | 0.77 | 11 | 0.28 | 130 | 0.80 | −8 | −12 to −4 |
| 18–49 | 167 | 1.43 | 106 | 0.94 | 80 | 0.73 | 55 | 0.50 | 408 | 0.91 | −7 | −8 to −5 |
| 50–64 | 73 | 1.63 | 75 | 1.44 | 78 | 1.37 | 48 | 0.84 | 274 | 1.30 | −4 | −6 to −2 |
| ≥65 | 47 | 1.25 | 46 | 1.15 | 64 | 1.46 | 54 | 1.07 | 211 | 1.23 | −1 | −4 to 1 |
| All age groups | 448 | 1.45 | 351 | 1.35 | 331 | 1.25 | 231 | 0.85 | 1361 | 1.29 | −4 | −3 to −5 |
*Mean annual relative change in incidence calculated by Poisson regression or negative binomial regression.
NA, not applicable.
The mean annual rates of all bacterial meningitis ranged from 1.97 in 1996 to 0.70 cases/100 000 person-years in 2014, with an annual decrease of 4% (95% CI −3% to −5%, table 1). During 2004–2014, 65 patients died within 30 days from culture (CFP, 10% (65/633)). There was no change in 30-day CFP from 2004–2009 (11% (43/402) to 2010–2014 (10% (22/231), p=0.22.
Characteristic of bacterial meningitis by age group
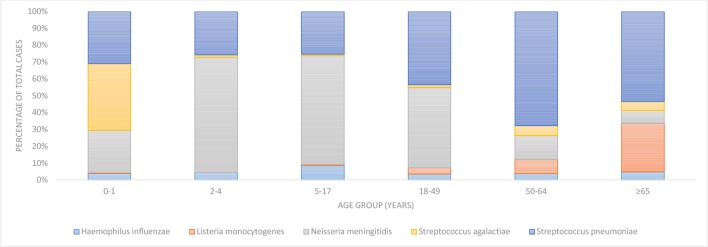
Children <2 years of age accounted for 20% of cases (268/1361) and had the highest incidence rate (11.38 cases/100 000 person-years, table 1). The most common pathogens in this age group were S. agalactiae (4.50 cases/100 000 person-years) and S. pneumoniae (3.52 cases/100 000 person-years, figure 1). From 1995 to 2014, the rate of bacterial meningitis in this age group decreased by 2% annually (95% CI −4% to −1%, table 1). The average 30-day CFP in 2004–2014 was 2% (3/140). In children 2–4 years of age, 70 cases (5%) of bacterial meningitis were reported during 1995 to 2014 (1.94 cases/100 000 person-years). The most common pathogens in this age group were N. meningitidis (1.33 cases/100 000 person-years) and S. pneumoniae (0.50 cases/100 000 person-years, table 1). During the study period, the rate of all meningitis did not change significantly (table 1). The 30-day CFP in 2004–2014 was 14% (4/128); all four deaths were due to N. meningitidis.
Figure 1.
Proportions of bacterial meningitis cases caused by five pathogens according to age group, Finland, 1995–2014.
Children 5–17 years of age accounted for 130 cases (9%) of bacterial meningitis and had the lowest rate (0.80 cases/100 000 person-years, table 1). N. meningitidis and S. pneumoniae were the main causes (0.52 and 0.20 cases/100 000 person-years, respectively, figure 1). From 1995 to 2014, the rate of bacterial meningitis decreased by 8% annually (95% CI −12% to −4%, table 1). The 30-day CFP was 7% (3/45); all three fatal cases were due to N. meningitidis.
Adults 18–49 years of age accounted for 408 cases (30%) of bacterial meningitis (0.91 cases/100 000 person-years, table 1). N. meningitidis and S. pneumoniae caused most of the cases (figure 1), with incidence rates 0.43 and 0.40 cases/100 000 person-years, respectively. During 1995–2014, the overall rate decreased by 7% annually (95% CI −8% to −5%, table 1). The 30-day CFP was 8% (13/152), with nine deaths due to S. pneumoniae infection.
Among persons 50–64 years of age, there were 274 cases (20%) of bacterial meningitis (1.30 cases/100 000 person-years, table 1), of which 186 cases (68%) were caused by S. pneumoniae (0.88 cases/100 000 person-years, figure 1). During the study period, the overall rate decreased by 4% annually (95% CI −6% to −2%, table 1). The 30-day CFP was 13% (18/143), with most fatal cases attributable to S. pneumoniae (16 deaths).
In adults ≥65 years of age, there were 211 cases (15%) of bacterial meningitis (1.23 cases/100 000 person-years, table 1). S. pneumoniae caused 53% (113/211) of the cases (0.66 cases/100 000 person-years), followed by L. monocytogenes. There was no significant change in the overall rate during 1995–2014 (table 1). This age group had the highest 30-day CFP (19%, 24/125). Half of the fatal cases were due to S. pneumoniae (12 deaths); L. monocytogenes caused 10 deaths.
Causes of bacterial meningitis
Streptococcus pneumoniae
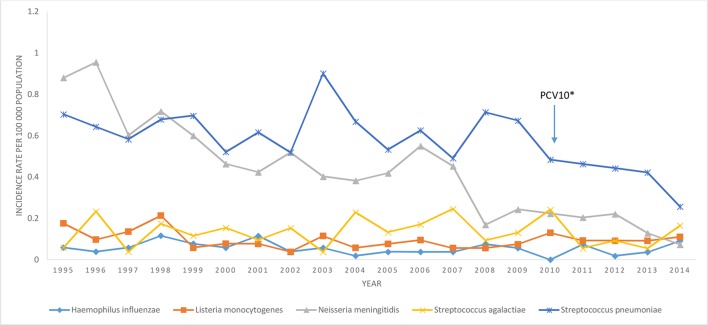
From 1995 to 2014, 611 cases of pneumococcal meningitis were reported. Median age was 48 years; 57% of cases were male (male to female IRR, 1.4 95% CI 1.2 to 1.6, table 2). The overall annual rate per 1 00 000 person-years decreased from 0.70 in 1995 to 0.26 in 2014 (figure 2), a 2% annual decrease (95% CI −4% to −1%, table 1).
Table 2.
Characteristics of bacterial meningitis cases, Finland, 1995–2014
| Characteristics | S. pneumoniae | N. meningitidis | S. agalactiae | L. monocytogenes | H. influenzae | Total |
| Gender, no of cases (% of total) |
||||||
| Male | 347 (57) | 268 (60) | 70 (50) | 71 (70) | 28 (48) | 784 (58) |
| Female | 264 (43) | 182 (40) | 71 (50) | 30 (30) | 30 (52) | 577 (42) |
| Age (years) | ||||||
| Median | 48 | 18 | 0 | 68 | 29 | 36 |
| IQR | 28–62 | 4–35 | 0 | 56–74 | 6–54 | 5–58 |
| Case fatality* | ||||||
| No of deaths (no of cases) | 38 (308) | 14 (163) | 2 (86) | 11 (50) | 0 (26) | 65 (633) |
| Case fatality proportion (%) | 12.3 | 8.6 | 2.3 | 22 | 0 | 10.3 |
*Data are for cases reported during 2004–2014.
Figure 2.
Incidence rate (per 100 000 person-years) of bacterial meningitis by year and pathogen, Finland, 1995–2014.
The incidence of pneumococcal meningitis decreased annually by 4% (95% CI −7% to 0%), 7% (95% CI −13% to −1%) and 4% (95% CI −6% to −1%) in age groups <2 years, 5–17 years and 18–49 years, respectively. During 2004–2014, S. pneumoniae accounted for 58% (38/65) of fatal cases (30-day CFP 12%, 38/308).
Of the 308 pneumococcal meningitis cases reported during 2004–2014, information on serotype was available for 296 (96%). The proportion of cases caused by PCV10 serotypes decreased from 61% (35/57) in 2004–2005 to 15% (9/36) in 2013–2014. PCV13 serotypes accounted for 70% (40/57) cases in 2004–2005 and 44% (16/36) in 2013–2014. In children less than 2 years, proportion of meningitis cases caused by PCV10 serotypes decreased from 75% (9/12) in 2004–2005 to 20% (1/5) in 2013–2014. In 2014, no meningitis cases were caused by PCV10 serotypes.
Neisseria meningitidis
During the study period, meningococcal meningitis accounted for 450 cases (0.43 cases/100 000 person-years) (table 1). Median age was 18 years and 60% of cases were male (male to female IRR 1.5, 95% CI 1.3 to 1.9, table 2). The overall annual incidence per 1 00 000 person-years decreased from 0.88 in 1995 to 0.07 in 2014; the annual decrease was −9% (95% CI −7% to −10%, table 1). The decline occurred in all age-groups except in <2 years and ≥65 years of age. The incidence decreased annually by 6% (95% CI −1% to −10%), 8% (95% CI −3% to −14%), 10% (95% CI −8% to −13%) and 12% (95% CI −8% to −13%) in age groups 2–4 years, 5–17 years, 18–49 years and 50–64 years, respectively. The overall 30-day CFP was 9% (14/163) and ranged from 3% (1/29) among children aged 0–1 years to 21% (4/19) among children aged 2–4 years.
During 2004–2014, information on N. meningitidis serogroups was available for 99% of cases (161/163). Serogroup B accounted for 85% (137/161) of isolates, C 11% (17/161) and Y 4% (7/161). In children <2 years, serogroup B caused 96% (26/27) of cases. MCV-4 and MenB vaccine serogroups caused 15% (24/161) and 85% (137/161) of all cases, respectively.
Haemophilus influenzae
From 1995 to 2014, 58 cases of H. influenzae were reported (0.06 cases/100 000 person-years, table 1). Median age was 29 years and male to female IRR was 1.0 (95% CI 0.6 to 1.7, table 2). The incidence rate ranged from 0.0 cases per 1 00 000 person-years in 2010 to 0.25 cases in 2007 (figure 2). Rates in all age groups were stable. From 2004 to 2014, there were no deaths due to H. influenzae.
In 2004–2014, non-encapsulated H. influenzae accounted for 69% (18/26), serotype f 23% (6/26) and type b 8% (2/26) of isolates.
Streptococcus agalactiae
Infection with S. agalactiae accounted for 141 cases of meningitis (0.13 cases/100 000 person-years), including 24 early-onset cases and 78 late-onset cases (table 1). The median age of cases was 30 days; male to female IRR was 1.03 (95% CI 0.7 to 1.4) (table 2). During the study period, annual rates ranged from 0.06 cases/100 000 person-years in 1995 to 0.17 cases in 2014 (figure 2), but overall rates of S. agalactiae did not change significantly (p=0.97, table 1). During 2004–2014, the 30-day CFP was 2% (2/86).
Listeria monocytogenes
During the study period, L. monocytogenes caused 101 cases of meningitis (0.13 cases/100 000 person-years), mostly among elderly persons (median age, 68 years). Of cases, 70% were men (male to female IRR 2.5, 95% CI 1.6 to 3.9, table 2). Overall incidence rates of Listeria meningitis did not vary significantly during the study period, ranging from 0.04 to 0.21/100 000 person-years (table 1). The overall 30-day CFP was 22% (11/50) and 28% (10/36) in persons ≥65 years of age.
Discussion
During 1995–2014, the most common causes of bacterial meningitis in Finland were S. pneumoniae and N. meningitidis. However, contribution of specific pathogens to the disease burden varied substantially by age. As in other developed countries, S. agalactiae was the most common cause of bacterial meningitis in children <1 years of age.6 The mean age of cases increased significantly during the study period mainly because of the decrease in incidence in children associated with PCV10 programme and declining secular trend in meningococcal meningitis.
During the study period, significant declines were seen in overall incidence of bacterial meningitis—primarily due to decreases in rates of N. meningitis and S. pneumoniae. Of interest, the decrease in incidence of N. meningitidis was greater than for pneumococcal meningitis, although there is no routine vaccination programme for meningococcal disease in Finland. Changes in rates of meningococcal disease have also been observed in other countries in Europe and worldwide.16 17 The reasons for these declines in incidence are not clear but may be related to population immunity to circulating strains, changes in colonising organisms in the nasopharynx or increasing use of influenza vaccine. Also, changes in behavioural risk factors such as lower prevalence of smoking or crowding might contribute.18 19 In some countries, decreases were related to meningococcal vaccination. After the introduction of conjugate serogroup C meningococcal vaccine, vaccine serogroup disease nearly disappeared in England20 and the Netherlands.21 Direct and indirect (herd protection) vaccine effects were also reported from other European countries including Spain, Ireland and Belgium.22 23 Immunisation of high risk groups with recently licensed protein-based vaccines targeted against meningococcal serogroup B might also be considered in Finland. However, updated cost-effective analysis is needed for decision-making about introduction of meningococcal vaccination programs.
Before the introduction of PCV10, considerable variation in pneumococcal meningitis incidence rates was seen. As there were no major changes in surveillance or diagnostic practices in Finland, these changes may be related to emergence of new serotypes, selective pressure from antibiotic use or natural fluctuation in serotypes.24–26 The decline in pneumococcal meningitis incidence in children <2 years of age was associated with introduction of PCV10 in the NVP in 201015; PCV10 serotypes in this age group were significantly reduced and by 2014 no vaccine serotype meningitis cases were reported. In vaccine-eligible children, the overall rate of pneumococcal meningitis was reduced by 46% as a result of a 69% reduction in PCV10-type meningitis.15 Many studies in USA and Europe have also documented significant declines in the incidence of pneumococcal meningitis in both vaccinated and unvaccinated groups after introduction of PCV programmes.11 12 27–29 In Finland, it might be possible to achieve further reductions with higher valency conjugate vaccine formulations.
The incidence rate of L. monocytogenes, N. meningitidis and S. pneumoniae was higher in men than women. L. monocytogenes meningitis cases were 2.5 times more likely to be men. Higher rates of listeriosis in males have also been observed in other studies.7 However, the reasons are unknown, but may be related to higher prevalence of underlying conditions, alcoholism among men and liver diseases (including alcoholic cirrhosis).30 In pneumococcal and meningococcal meningitis, possible reasons may be higher prevalence of underlying medical conditions, smoking and alcoholism.31 As listeriosis is primarily transmitted through contaminated food, important prevention efforts include health education about dietary guidelines for high risk groups, such as pregnant women and the elderly.32
The overall 30-day CFP for meningitis did not change significantly during 1995–2014. However, the unchanged CFP may be related to the altered age distribution of cases. Older age is associated with higher risk of poor outcome.33 In addition, pathogen distribution has changed and the case fatality for meningococcal meningitis is lower compared with pneumococcal meningitis. The small number of fatal cases in our study did not allow assessing changes in CFP by age group and pathogen. The 30-day CFP was highest for L. monocytogenes (22%), which is comparable with results from the Netherlands and Spain.7 34 Most of the fatal cases of bacterial meningitis in persons ≥50 years were attributable to S. pneumoniae. Cases who had pneumococcal meningitis were older than those who were infected with other encapsulated bacteria and likely had higher prevalence of comorbidities increasing the risk of pneumococcal infection and poor outcome.35 Because of lack of clinical data, we could not assess the potential impact of treatment changes, such as dexamethasone use, on case fatality. The relatively high CFP emphasises the importance of immediate initiation of treatment and supportive care after diagnosis to improve outcome of bacterial meningitis.
As expected, H. influenzae was the least common cause of bacterial meningitis. However, the stable number of cases over 20 years suggest existence of small group of individuals with risk factors for H. influenzae (such as chronic respiratory disease and impaired immunity).36 Conjugate vaccination has nearly eliminated Hib meningitis in many high-income countries.37 38 However, changes in the epidemiology of invasive H. influenzae have been observed and currently most cases occur in adults39 and non-encapsulated, non-typable H. influenzae have dominated since 2004.
Because the data on laboratory confirmed cases are transmitted electronically directly from the clinical microbiology laboratories’ database to the national surveillance database, a strength of our study is comprehensive case ascertainment. In addition, almost all isolates of N. meningitidis, H. influenzae and S. pneumoniae (98%) were available for serotyping/grouping at THL reference laboratory. However, our study has several limitations. As the data were from laboratory-based surveillance system, information on clinical presentation or treatment was not available. Therefore, culture-negative meningitis cases diagnosed on the basis of clinical symptoms and findings were not included in the analysis dataset. In addition, cases diagnosed by PCR or antigen detection were not included. As CSF cultures are negative in 11%–30% of patients with bacterial meningitis,40 the total number of meningitis cases is underestimated. Another limitation is that NIDR database does not include information on the cause of death. However, most of deaths associated with bacterial meningitis occur early (within 14 days of admission), suggesting that they were related to the infection.41
In conclusion, this study describes the epidemiological characteristics of >1300 cases of bacterial meningitis reported to national surveillance over 20 years. It documents the sustained population impact of infant conjugate vaccination against Hib and introduction of PCV on reducing burden of bacterial meningitis as well as decline in meningococcal meningitis due to secular trend. However, disease burden had shifted to older people and no changes in the overall proportion of fatal cases were seen. Data on changes in causative organisms and age distribution for meningitis cases are important for evaluating clinical guidelines for empirical antibiotic therapy in bacterial meningitis. Continued epidemiological surveillance is necessary to monitor changing trends and serotype distribution, assessing the impact of vaccination programs and developing future vaccination strategies.
Supplementary Material
Footnotes
Contributors: Study concept and design: AP, OL, PN. Acquisition of data: MT, JO, OL, PN. Analysis and interpretation of data: AP, MT, JO, OL, PN. Drafting of the manuscript: AP, PN. Critical revision of the manuscript for important intellectual content: AP, MT, JO, OL, PN. Statistical analysis: AP, JO. Obtained funding: PN. Study supervision: PN. Final approval: AP, MT, JO, OL, PN.
Funding: This study was supported by the School of Health Sciences, University of Tampere and the National Institute for Health and Welfare (THL) in Helsinki, Finland.
Competing interests: None declared.
Ethics approval: Data used in the analysis were collected as a part of surveillance and infection control activities which falls under the existing mandate of the National Institute for Health and Welfare (THL).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Edmond K, Clark A, Korczak VS, et al. . Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2010;10:317–28. 10.1016/S1473-3099(10)70048-7 [DOI] [PubMed] [Google Scholar]
- 2. Sáez-Llorens X, McCracken GH. Bacterial meningitis in children. Lancet 2003;361:2139–48. 10.1016/S0140-6736(03)13693-8 [DOI] [PubMed] [Google Scholar]
- 3. de Jonge RC, van Furth AM, Wassenaar M, et al. . Predicting sequelae and death after bacterial meningitis in childhood: a systematic review of prognostic studies. BMC Infect Dis 2010;10:232 10.1186/1471-2334-10-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010;23:467–92. 10.1128/CMR.00070-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim KS. Acute bacterial meningitis in infants and children. Lancet Infect Dis 2010;10:32–42. 10.1016/S1473-3099(09)70306-8 [DOI] [PubMed] [Google Scholar]
- 6. Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine 2013;31 Suppl 4:D7–D12. 10.1016/j.vaccine.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 7. Aouaj Y, Spanjaard L, van Leeuwen N, et al. . Listeria monocytogenes meningitis: serotype distribution and patient characteristics in the Netherlands, 1976-95. Epidemiol Infect 2002;128:405–9. 10.1017/S0950268802006969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nau R, Djukic M, Spreer A, et al. . Bacterial meningitis: an update of new treatment options. Expert Rev Anti Infect Ther 2015;13:1401–23. 10.1586/14787210.2015.1077700 [DOI] [PubMed] [Google Scholar]
- 9. van de Beek D, Brouwer MC, Thwaites GE, et al. . Advances in treatment of bacterial meningitis. Lancet 2012;380:1693–702. 10.1016/S0140-6736(12)61186-6 [DOI] [PubMed] [Google Scholar]
- 10. Fitch MT, van de Beek D. Emergency diagnosis and treatment of adult meningitis. Lancet Infect Dis 2007;7:191–200. 10.1016/S1473-3099(07)70050-6 [DOI] [PubMed] [Google Scholar]
- 11. Dery MA, Hasbun R. Changing epidemiology of bacterial meningitis. Curr Infect Dis Rep 2007;9:301–7. 10.1007/s11908-007-0047-7 [DOI] [PubMed] [Google Scholar]
- 12. McIntyre PB, O'Brien KL, Greenwood B, et al. . Effect of vaccines on bacterial meningitis worldwide. Lancet 2012;380:1703–11. 10.1016/S0140-6736(12)61187-8 [DOI] [PubMed] [Google Scholar]
- 13. Peltola H, Kilpi T, Anttila M, et al. . Rapid disappearance of Haemophilus influenzae type b meningitis after routine childhood immunisation with conjugate vaccines. Lancet 1992;340:592–4. 10.1016/0140-6736(92)92117-X [DOI] [PubMed] [Google Scholar]
- 14. Palmu AA, Jokinen J, Borys D, et al. . Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet 2013;381:214–22. 10.1016/S0140-6736(12)61854-6 [DOI] [PubMed] [Google Scholar]
- 15. Jokinen J, Rinta-Kokko H, Siira L, et al. . Impact of ten-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in finnish children--a population-based study. PLoS One 2015;10:e0120290 10.1371/journal.pone.0120290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Centre for Disease Prevention and Control. Surveillance of invasive bacterial diseases in Europe, 2012. Stockholm: ECDC 2015. [Google Scholar]
- 17. Cohn AC, MacNeil JR, Harrison LH, et al. . Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin Infect Dis 2010;50:184–91. 10.1086/649209 [DOI] [PubMed] [Google Scholar]
- 18. Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009;27(Suppl 2):B51–B63. 10.1016/j.vaccine.2009.04.063 [DOI] [PubMed] [Google Scholar]
- 19. Sadarangani M, Pollard AJ. Can we control all-cause meningococcal disease in Europe? Clin Microbiol Infect 2015;22:S103–12. [DOI] [PubMed] [Google Scholar]
- 20. Martin NG, Sadarangani M, Pollard AJ, et al. . Hospital admission rates for meningitis and septicaemia caused by Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae in children in England over five decades: a population-based observational study. Lancet Infect Dis 2014;14:397–405. 10.1016/S1473-3099(14)70027-1 [DOI] [PubMed] [Google Scholar]
- 21. Bijlsma MW, Bekker V, Brouwer MC, et al. . Epidemiology of invasive meningococcal disease in the Netherlands, 1960-2012: an analysis of national surveillance data. Lancet Infect Dis 2014;14:805–12. 10.1016/S1473-3099(14)70806-0 [DOI] [PubMed] [Google Scholar]
- 22. Trotter CL, Ramsay ME. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol Rev 2007;31:101–7. 10.1111/j.1574-6976.2006.00053.x [DOI] [PubMed] [Google Scholar]
- 23. Maiden MC, Ibarz-Pavón AB, Urwin R, et al. . Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis 2008;197:737–43. 10.1086/527401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klemets P, Lyytikäinen O, Ruutu P, et al. . Trends and geographical variation in invasive pneumococcal infections in Finland. Scand J Infect Dis 2008;40:621–8. 10.1080/00365540801938931 [DOI] [PubMed] [Google Scholar]
- 25. Harboe ZB, Benfield TL, Valentiner-Branth P, et al. . Temporal trends in invasive pneumococcal disease and pneumococcal serotypes over 7 decades. Clin Infect Dis 2010;50:329–37. 10.1086/649872 [DOI] [PubMed] [Google Scholar]
- 26. Black S. The Volatile Nature of Pneumococcal Serotype Epidemiology. Pediatr Infect Dis J 2009;1. [DOI] [PubMed] [Google Scholar]
- 27. Vestrheim DF, Løvoll O, Aaberge IS, et al. . Effectiveness of a 2+1 dose schedule pneumococcal conjugate vaccination programme on invasive pneumococcal disease among children in Norway. Vaccine 2008;26:3277–81. 10.1016/j.vaccine.2008.03.087 [DOI] [PubMed] [Google Scholar]
- 28. Hsu HE, Shutt KA, Moore MR, et al. . Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med 2009;360:244–56. 10.1056/NEJMoa0800836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsai CJ, Griffin MR, Nuorti JP, et al. . Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin Infect Dis 2008;46:1664–72. 10.1086/587897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liatsos GD, Thanellas S, Pirounaki M, et al. . Listeria monocytogenes peritonitis: presentation, clinical features, treatment, and outcome. Scand J Gastroenterol 2012;47:1129–40. 10.3109/00365521.2012.704935 [DOI] [PubMed] [Google Scholar]
- 31. Klemets P, Lyytikäinen O, Ruutu P, et al. . Invasive pneumococcal infections among persons with and without underlying medical conditions: implications for prevention strategies. BMC Infect Dis 2008;8:96 10.1186/1471-2334-8-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Institute for Health and Welfare, THL, Helsinki, Finland. https://www.thl.fi/en/web/infektiotaudit/taudit-ja-mikrobit/bakteeritaudit/listeria
- 33. Bodilsen J, Dalager-Pedersen M, Schønheyder HC, et al. . Dexamethasone treatment and prognostic factors in community-acquired bacterial meningitis: a danish retrospective population-based cohort study. Scand J Infect Dis 2014;46:418–25. 10.3109/00365548.2014.887223 [DOI] [PubMed] [Google Scholar]
- 34. Amaya-Villar R, García-Cabrera E, Sulleiro-Igual E, et al. . Three-year multicenter surveillance of community-acquired Listeria monocytogenes meningitis in adults. BMC Infect Dis 2010;10:324 10.1186/1471-2334-10-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. . Community-acquired bacterial meningitis in adults in the Netherlands, 2006-14: a prospective cohort study. Lancet Infect Dis 2016;16:1–9. 10.1016/S1473-3099(15)00430-2 [DOI] [PubMed] [Google Scholar]
- 36. Ladhani S, Slack MP, Heath PT, et al. . Invasive Haemophilus influenzae disease, Europe, 1996-2006. Emerg Infect Dis 2010;16:455–63. 10.3201/eid1603.090290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev 2000;13:302–17. 10.1128/CMR.13.2.302-317.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watt JP, Wolfson LJ, O'Brien KL, et al. . Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 2009;374:903–11. 10.1016/S0140-6736(09)61203-4 [DOI] [PubMed] [Google Scholar]
- 39. Dworkin MS, Park L, Borchardt SM. The changing epidemiology of invasive Haemophilus influenzae disease, especially in persons > or = 65 years old. Clin Infect Dis 2007;44:810–6. 10.1086/511861 [DOI] [PubMed] [Google Scholar]
- 40. Brouwer MC, Van De Beek D, Heckenberg SG, et al. . Meningitis in adults. Clin Infect Dis 2006;43:1233–8. [DOI] [PubMed] [Google Scholar]
- 41. McMillan DA, Lin CY, Aronin SI, et al. . Community-acquired bacterial meningitis in adults: categorization of causes and timing of death. Clin Infect Dis 2001;33:969–75. 10.1086/322612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.