Figure 1.
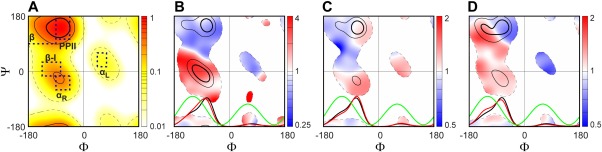
Backbone torsion angles distributions observed in our newly generated coil library, illustrated for (A) all residues, (B) 963 residues neighbored by two Gly (or subset {G‐X‐G}), (C) 11,938 residues followed by a Lys, Gln or Arg ({X‐K|Q|R}), and (D) 7,122 residues next to a Phe, Trp or Tyr ({F|W|Y‐X}). For each plot, three different φ/ψ conformational regions are marked as those with a normalized residue density d(ϕ,ψ)/d max above thresholds of 60%, 30%, and 3%, respectively, in the newly generated coil library or its subsets. Their boundaries are marked by dark solid, light solid and light dashed lines, respectively. The residue density, d(ϕ,ψ), is derived by convolution of each of the ϕk/ψk coil library entries with a Gaussian function, exp(–((ϕ – ϕk)2 + (ψ – ψk)2))/450).42 (A) Ramachandran density map of all residues in the coil library, d(ϕ,ψ)/d max; (B‐D) for each of the three subsets, the ratio of d(ϕ,ψ)/Σd(ϕ,ψ) between the subset and all other residues (center residue X ≠ Gly, Pro and Xaa‐Pro) is plotted from blue to red (B‐D). To illustrate the impact of different nearest‐neighbors on the ϕ torsion angle distribution, the normalized ϕ torsion angle distribution is also plotted (red) at the bottom of each plot (B‐D), together with the normalized ϕ angle distribution observed for all other residues in the coil library (black) and the scaled 3 J HN‐Hα Karplus equation curve (green). Dashed boxes mark secondary structure regions: β (–180°<ϕ<–90°, 90°<ψ<180°), PPII (–90°<ϕ<–45°, 105°<ψ<180°), αR (–90°<ϕ<–45°, −60°<ψ<−15°), type I β‐turn (β‐I) (–135°<ϕ<–75°, −15°<ψ<30°), and αL (45°<ϕ<75°, 15°<ψ<60°) (see labels in A).
