Abstract
Restorative proctocolectomy (RPC) with ileal pouch-anal anastomosis (IPAA) is the standard surgical treatment for ulcerative colitis (UC). Emergency colectomies are performed for fulminant colitis (ie, toxic megacolon, profuse bleeding, perforation, or sepsis). The RPC and IPAA involve manipulation of the proximal ileum, which may influence the essential physiological function of gut-associated lymphoid tissues. Circulating plasma immunoglobulin G (p-IgG) deficiency is observed in patients with fulminant UC. In addition, increased levels have been reported in colonic tissues of active UC compared with quiescent disease. We aimed to examine levels of p-IgG for clinical evaluation following emergency colectomies in patients with fulminant UC compared with patients with quiescent disease having elective RPC operations. In total 45 patients received an ileoanal pouch (IAP) due to UC. In all, 27 patients were men and 18 were women. The mean age was 34 years (range: 18-55). Because of fulminant UC, 26 patients had emergency subtotal colectomies with terminal ileostomy (TI). During second operation, the rectum was excised, and an IAP with diverting loop ileostomy (DLI) was performed. Nineteen patients had elective operations and had colectomies performed in conjunction with the pouch operation. Mucosectomy was performed in all groups. As a last procedure, the DLI was closed. Blood samples for immunoglobulin G (IgG) analyses were collected from each patient before the colectomy, after the colectomy with TI (before construction of the pouch), during the period with pouches (prior to DLI closure), and at 1, 2, and 3 years and at mean 13.7 years (range: 10-20) after DLI closure. Immunoglobulin G was determined by immunonephelometric assay technique. The statistics were analyzed by analysis of variance and linear regression. Preoperatively, p-IgG was significantly lower in the patients who had emergency operations compared with the group that had elective operations, 9.9 ± 3.0 vs 11.5 ± 3.3 g/L (P < .03). During the manipulative period with TI and/or DLI, the p-IgG levels were increased in both points, but the increase was not statistically significant (P = .26 and P = .19). During functional IAP at 1, 2, and 3 years and at mean 13.7 years (range: 10-20), there was a statistical increase in p-IgG levels (P < .002, P < .005, P < .005, and P < .0001) compared with preoperative levels. These changes did not correlate with episodes of pouchitis (P = .51). In patients having elective operations, p-IgG did not change preoperatively. After 12 months with functional pouches, the p-IgG levels were similar in both groups to the elective patient group preoperatively. In conclusion, p-IgG was found to be significantly lower in the emergency surgery patients compared with the elective surgery group preoperatively. This difference was probably due to increased losses and impaired gut lymphoid tissue production of IgG in the acute fulminant phase of UC. After 12 months of DLI closure, significant differences were no longer found between the emergency and elective surgery groups. Restoration and increased p-IgG levels after RPC would be due to an exaggerated response to make up for lower precolectomy values and may be interpreted as a rehabilitation biomarker.
Keywords: Immunoglobulin G, ulcerative colitis, fulminant colitis, emergency colectomy, elective colectomy, terminal ileostomy, proctocolectomy, pelvic pouch, mucosectomy, diverting loop ileostomy
Introduction
The actual biopathogenesis of ulcerative colitis (UC) remains unknown.1 However, UC is considered a disease of immune-mediated gut disorder by dysregulated innate and adaptive immunities.1,2 Total proctocolectomy cures a patient of the intestinal manifestation of chronic UC.3 The timing of surgery during the illness will influence the optional choice of operation, the frequency of subsequent complications, and the functional outcomes. There are 2 definitive approaches to surgery for UC treatment: emergency procedures and elective procedures.3 Either option involves skillful manipulation of the distal ileum, which may influence the essential physiological function of ileal mucosa-associated lymphoid tissue of the gastrointestinal immune system.4 Gut-associated lymphoid tissue is one of the major subdivisions of the complement system, which is an important part of the innate immune defense system.5–8 Immunoglobulin G (IgG) antibodies are the most abundant type of antibody protein (75%-80%) found in all bodily fluids.9 The IgG antibodies are critically important in fighting pathogens.5,10 The IgG concentration in patients with UC has been shown to be significantly altered11 due to markedly increased overproduction of IgG by intestinal mononuclear cells.12 In this study, and in our previous observation,13 the reverse is true. We did not see an increase, but rather a decrease, in circulating total p-IgG in patients with fulminant UC prior to emergency colectomy.
Because of fulminant UC refractory to medical treatment, emergency colectomy is indicated.14–16 The initial operation is a subtotal colectomy.17 The rectum remains, and a terminal ileostomy (TI) is constructed. After subtotal colectomy with TI, the disease and symptoms associated with the disease are effectively eliminated.3 At this time, patients are able to discontinue all of their immunosuppressive medications, and their overall health is quickly restored. In a second operation stage, the remaining rectum is excised, and a pouch is constructed from the distal ileum to replace the rectum. A diverting loop ileostomy (DLI) is then constructed to divert the bowel contents from the proximal part of the small intestine to allow healing of the anastomosis and the pouch. Elective patients receive the same operation, but the colon is excised simultaneously.17 The elective surgery is indicated in patients with chronic UC due to response failure of medical management to control symptoms, complications associated with side effects of medications, stricture formation, mucosal dysplasia, and dysplasia-associated lesion or mass, malignancy, or extraintestinal manifestations.3,18
In this study, all patients received a DLI. A DLI not only changes the mucosal appearance of the bowel19,20 but also changes bacterial content quantitatively and qualitatively.21–23 As a last step in the procedure, the DLI is closed, the distal small bowel and the pouch are put into function, and the most distal part of the small intestine becomes a functional pouch, specifically, a reservoir/container for the bodily waste excrement. The present investigation aims to (1) determine whether the bowel manipulations during restorative proctocolectomy and ileal pouch-anal anastomosis (IPAA) change the circulating p-IgG pattern concentration, (2) evaluate the post-UC disease activity, and (3) evaluate possible differences in the p-IgG saturation between the emergency and elective surgery patients, before and after surgery and with acceptably functional pouches, over a 10- to 20-year period.13,24
Methods
The STARD (Standards for Reporting of Diagnostic Accuracy) statement: To facilitate the completeness and transparency of our biomarker study, and avoid risk of bias, we used the STARD approach.25,26 All STARD materials, including the checklist (record last updated February 7, 2017), are available at http://www.equator-network.org/reporting-guidelines/stard.25
Ethical Considerations
The study was conducted in accordance with the second Helsinki Declaration27 and approved by the Meharry Medical College and Vanderbilt University Medical Center (USA) and Karolinska Institutet (Sweden) Institutional Ethical Committees. Informed consent was given by all patients, and patient participation in the study was voluntary.
Patients and Operation Procedures
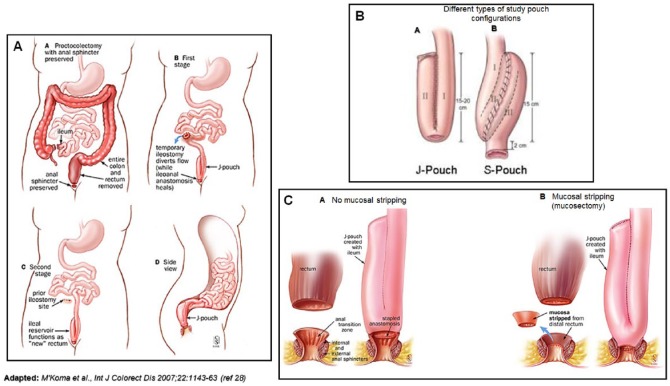
In total, 15 patients, 27 men and 18 women, were enrolled in the study. The mean age was 34 years (range: 18-55). In all, 26 patients initially had subtotal colectomies with TI and pouch construction during a later operation, whereas 19 had their colectomies and pouch constructions simultaneously. Emergency surgery patients had procedures because of failing conservative management of UC (Figure 1AA). The elective group, of which 8 patients had dysplasia and 1 had adenocarcinoma (Dukes A),29 had operations due to chronic, continuous UC (Figure 1AA-B). The overall duration of the subtotal colectomies in the emergency group was 4.8 years (range: 0-19), and in the elective surgery patients, it was 11.9 years (range: 1.5-31). Patients with subtotal colectomies kept their TIs for a mean of 7.7 months (range: 3-22) before the pouch construction procedure. A DLI was constructed in all patients during the latter operation (Figure 1AC) and retained for a mean period of 7 months (range: 2-27). The excluded terminal ileum during the DLI was a mean length of 96 cm (range: 57-133), including the small bowel used for the pouch construction. Of the 45 patients, 29 received S pouches (mean: 3 cm × 10 cm), and 16 received J pouches (mean: 2 cm × 20.5 cm). All of the pouches and anastomosis manipulations were handsewn.
Figure 1.
Restorative proctocolectomy and ileoanal pouch anastomosis. (AA-D) Two-stage procedure of restorative proctocolectomy: (AA) proctocolectomy with anal sphincter preserved, (AB) first stage, (AC) second stage, and (AD) side view. (B) Different pouch configurations in the study (J-shaped and S-shaped reservoirs). (C) J-shaped reservoir: (CA) ileal pouch-anal anastomosis without mucosectomy (double-stapled technique) and (CB) with mucosectomy.
Adapted with permission from M’Koma et al.28
Overnight-fasted patients appeared for p-IgG analyses: (1) before colectomy; (2) after colectomy with TI, but before pouch construction (for the emergency group); (3) with ileal pouch while having DLI; and (4) with functional pouches at 1, 2, and 3 years and at mean 13.7 years (range: 10-20) after DLI closure. Blood specimens were collected in the mornings before breakfast, and p-IgG levels were analyzed and determined by means of a commercial enzyme-linked immunosorbent assay. The fasting reference values for p-IgG are 7 to 15 g/L. The reference values are derived from data that are traditionally verified, usually every 2 years, by comparing p-IgG levels of up to 100 healthy control volunteers.
Statistics
The Biomedical Data Processing Package (BMDP; Statistical Software Inc., Los Angeles, CA, USA; 1986) was used to perform statistical calculations.30 This statistical software was used to estimate the total numbers of surgeries and their variances for each year. Distributed data were compared from the values (1) before colectomy, (2) after colectomy with TI, (3) after IPAA with a DLI, and (4) at 1 to 13.7 years (range: 10-20) after DLI closure by analysis of variance method with Bonferroni correction for multiple comparisons.31,32 The correlation was calculated using the Pearson product-moment correlation coefficient.33 Comparable test results for length of excluded ileum and plasma variables were accomplished with linear regression analysis. Differences were regarded as significant for a probability value of less than .05, unless it was stated otherwise.
Results
Surgical manipulation procedures are depicted in Figure 1, and p-IgG variables are depicted in Table 1. Observations prior to colectomy showed that the total p-IgG levels in patients with emergency colectomies were significantly depleted compared with the elective surgery group: 9.9 ± 3.0 vs 11.5 ± 3.0 g/L (P < .03) (Table 1). During the manipulative period with TI and DLI, the p-IgG levels were increased in both groups, but the increase was not statistically significant (P = .26 and P = .19). During the functional period, the analyses of the emergency surgery patients at 1, 2, and 3 years and at mean 13.7 years (range: 10-20) of functional ileoanal pouch (IAP) showed that there was a statistically significant, steady increase from precolectomy levels of 9.9 ± 3.0 to 11.6 ± 3.0 g/L (P < .002), 13.0 ± 0.65 g/L (P < .005), 13.5 ± 2.6 g/L (P < .005), and 15 ± 4.2 g/L (P < .0001), respectively. A positive and significant correlation was noted in the emergency surgery patients (r2 = .78; P < .001) during the period following pouch surgery. The significantly increasing levels were seen 12 months following closure of a DLI and continued to increase gradually throughout the study. In the patients who had elective operations, the data measurements of p-IgG concentration were identical (P = NS) throughout the observation. There was no correlation (P = NS) between the steadily increasing p-IgG levels with respect to (1) patient age, (2) patient sex, (3) duration of subtotal colectomy prior to and after colectomy with TI, (4) duration and length of the diverted ileum while having DLI, or (5) episodes of pouch inflammation (pouchitis) or rectal cuff inflammation (cuffitis). Because mucosectomy was performed in all patient groups, a comparison between patients with and without mucosectomy on p-IgG levels during the surveillance follow-up was not done.
Table 1.
Number of patients with ulcerative colitis with surgery, indication to colectomy (emergency or elective), type of pouch, type and mean (range) duration and length of excluded ileum while having DLI.
| Precolectomy phase metrics |
Pouches: no. and type of ileal reservoir, duration and length of the excluded ileum while having DLI |
|||||||
|---|---|---|---|---|---|---|---|---|
| Operation base | No. of colectomy patients | No. of pathologic patients (%) | Mean ± SD, g/L | TI time, mean (range), mo | No. and type of reservoir, S-shaped and J-shaped | DLI duration time, mean (range), mo | Length of excluded ileum during DLI, mean (range), cm | |
| Emergency | 26 | 2 (8) | 9.9 ± 3 | — | 13 6 | 6.0 (2-12) | 98.0 (60-133) | |
| Elective | 19 | 2 (11) | 11.5 ± 3 | 7.7 (3-22) | 16 10 | 7.0 (2-27) | 94.0 (57-130) | |
| P value | .03 | — | — | — | — | |||
| Manipulative phase metrics | ||||||||
| Operation base | TI |
DLI |
||||||
| No. of colectomy patients | No. of pathologic patients (%) | Mean ± SD, g/L | P value compared with precolectomy | No. of colectomy patients | No. of pathologic patients (%) | Mean ± SD, g/L | P value compared with precolectomy | |
| Emergency | 26 | 1 (4) | 10.4 ± 3 | .26 | 26 | 2 (8) | 10.7 ± 3 | .19 |
| Elective | 19 | NA | NA | NA | 19 | 2 (11) | 11.8 ± 3 | .55 |
| P value | NA | .15 | ||||||
| Functional phase metrics (years of follow-up after DLI closure) | ||||||||
| Operation base | 1 y of functional IAP (short-term 1) |
2 y of functional IAP (short-term 2) |
||||||
| No. of colectomy patients | No. of pathologic patients (%) | Mean ± SD, g/L | P value compared with precolectomy | No. of colectomy patients | No. of pathologic patients (%) | Mean ± SD, g/L | P value compared with precolectomy | |
| Emergency | 26 | 3 (16) | 11.6 ± 3 | .002 | 26 | 0 (0) | 13.0 ± 0.65 | .005 |
| Elective | 19 | 2 (11) | 11.9 ± 3 | .45 | 19 | 0 (0) | 12.2 ± 0.77 | .07 |
| P value | .39 | .38 | ||||||
| Operation base | 3 y of functional IAP (short-term 3) |
13.7 y (range: 10-20) of functional IAP (long-term) |
||||||
| No. of colectomy patients | No. of pathologic patients (%) | Mean ± SD, g/L | P value compared with precolectomy | No. of colectomy patients | No. of pathologic patients (%) | Mean ± SD, g/L | P value compared with precolectomy | |
| Emergency | 26 | 1 (4) | 13.0 ± 2.6 | .005 | 26 | 1 (4) | 15.42 ± 0.98 | .0001 |
| Elective | 19 | 0 (0) | 12.0 ± 0.9 | .42 | 19 | 0 (0) | 12.2 ± 0.77 | .54 |
| P value | .54 | .005 | ||||||
Abbreviations: DLI, diverting loop ileostomy; IAP, ileoanal pouch; NA, not applicable; TI, terminal ileostomy.
Comparison of mean ± SD of plasma immunoglobulin G (g/L) circulating level between elective vs emergency surgery patients, before proctocolectomy, while having DLI and after 1, 2, 3, and 13.7 years (range: 10-20) of functional pouch after diverting loop ileostomy closure.
Discussion
To date, the exact pathogenesis of UC remains to be elucidated.1,2 The normal intestinal mucosal immune system is constantly stimulated by the luminal contents,11 which strongly suggests that luminal IgG subclasses are not randomly expressed.12 The IgG subclass deficiencies are shown to be associated with an increased susceptibility to infection.34 Most of the patients have a regulatory dysfunction,34 and the deficiencies are most often relative rather than absolute.34,35 In UC, elevation of circulating IgG above reference level suggests a pathway of bowel inflammatory reaction and tissue injury in the intestine.11 Although p-IgG deficiencies have been extensively studied,8 very limited data are available regarding clinical outcomes in humoral immunodeficiency. Previous studies reported that total p-IgG levels in patients with UC were significantly increased, for both active and inactive diseases, and concluded that in such scenarios, p-IgG could be used as additional markers of the disease.7,12 Interestingly, in our previous experience,13 and in this observation, a significant difference was discovered in mean p-IgG prior to colectomy between emergency surgery patients with fulminant UC compared with those who had elective operations because of quiescent UC. Total p-IgG was found to be significantly depleted (P < .03) in patients with fulminant UC.13 Similar observations were also reported by other research clinicians.36 Our interpretation of this depletion is that despite preferentially increased production of luminal IgG during the antigen vs antibody reaction against the gut mucosal resistance, acute severe ulceration causing diarrhea led to significant loss of IgG. The increased losses affect absorption of gut IgG during active UC. Therefore, a depletion of circulating p-IgG baseline is inevitable. Impaired production of IgG due to disease fulmination could be another possible explanation.
During the manipulative period with the TI and/or DLI, and during the functional period, no statistically significant differences of p-IgG were noted in patients who had elective operations for quiescent disease. This indicates that the manipulation of the ileum and the changes in gut bacterial flora21–23 did not affect the IgG production. However, in the emergency surgery group, the p-IgG concentrations were equal to preoperative levels during the manipulative period but significantly increased during functional IAP. These observations of increased p-IgG levels may seem to indicate mucosal immune system functional restoration,11 to an exaggerated response to make up for lower precolectomy values, hence, an indication of patient improvement and/or rehabilitation. For future therapeutic strategies that may include using immunoglobulin levels in patients with UC as a prognostic indicator or boosting humoral immunity as a treatment, we need to gain an understanding of immune and inflammatory regulatory responses in a variety of clinical conditions within the intestine.
Summary
Preoperatively, patients who had emergency operations due to fulminant UC showed significantly lower concentrations of p-IgG when compared with patients with quiescent UC who had elective operations. The low p-IgG concentration in the emergency surgery patients with active disease may have occurred as a result of increased losses and impaired production of IgG because of diseased gut luminal mucosa. The p-IgG levels steadily continued to increase after colectomy. Increased p-IgG did not correlate with episodes of pouchitis or cuffitis. Therefore, the restored and normalized p-IgG following colectomy surgery may be interpreted as a sign of patient rehabilitation rather than a mere immunopathophysiology biomarker. The p-IgG levels in patients with quiescent UC who had elective operations remained unchanged throughout the study.
Acknowledgments
The authors thank the Meharry Office for Scientific Editing and Publications for scientific editing support (NIH/S21MD000104); Meharry Medical College, Vanderbilt-Ingram Cancer Center and Tennessee State University: Partners in Eliminating Cancer Disparities U54CA163069; Meharry Clinical and Translational Research Center (MeTRC) grant # 2 U54 MD007593-08 funded by NIMHD/NIH; Vanderbilt University Medical Center, Department of Surgery, Colon and Rectal Surgery; Meharry Schools of Medicine (SOM) and Graduate Studies and Research (SOGSR) for the support.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was by supported by NIH/NIDDK-R21DK095186, VICTR-CTSA-1UL1RR024975-01, NIH/NCATS-VICTR-2UL1TR000445-06, NIH/NCI-3U54CA091408-09S1, and NIH/NCI-3U54CA091408-09S2.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: ATH: Acquisition of data, analysis and interpretation of data, critical revision of manuscript. JWU: Material and technical support, revision of the manuscript. AEM: Original idea of the paper, formulated the protocol and contribution to data abstraction and analysis. Critical drafting, writing and revision of the manuscript, statistical analysis and incorporated the comments from other authors and peer reviewers. Carried out the literature search, selection, and validity assessment.
All authors reviewed and approved the final manuscript.
References
- 1. Foersch S, Waldner MJ, Neurath MF. Innate and adaptive immunity in inflammatory bowel diseases. Dig Dis. 2013;31:317–320. [DOI] [PubMed] [Google Scholar]
- 2. Siegmund B, Zeitz M. Innate and adaptive immunity in inflammatory bowel disease. World J Gastroenterol. 2011;17:3178–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cima RR. Timing and indications for colectomy in chronic ulcerative colitis: surgical consideration. Dig Dis. 2010;28:501–507. [DOI] [PubMed] [Google Scholar]
- 4. Jansen A, Mandic AD, Bennek E, et al. Anti-food and anti-microbial IgG subclass antibodies in inflammatory bowel disease. Scand J Gastroenterol. 2016;51:1453–1461. [DOI] [PubMed] [Google Scholar]
- 5. Lubbers R, van Essen MF, van Kooten C, Trouw LA. Production of complement components by cells of the immune system. Clin Exp Immunol. 2017;188:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matar M, Rinawi F, Shamir R, Assa A. Hypergammaglobulinemia is a marker of extraintestinal manifestations in pediatric inflammatory bowel disease. Turk J Gastroenterol. 2017;28:131–134. [DOI] [PubMed] [Google Scholar]
- 7. Gouni-Berthold I, Baumeister B, Berthold HK, Schmidt C. Immunoglobulins and IgG subclasses in patients with inflammatory bowel disease. Hepatogastroenterology. 1999;46:1720–1723. [PubMed] [Google Scholar]
- 8. Horton N, Wu X, Philpott J, et al. Impact of low immunoglobulin G levels on disease outcomes in patients with inflammatory bowel diseases. Dig Dis Sci. 2016;61:3270–3277. [DOI] [PubMed] [Google Scholar]
- 9. Ardizzoni A, Manca L, Capodanno F, et al. Detection of follicular fluid and serum antibodies by protein microarrays in women undergoing in vitro fertilization treatment. J Reprod Immunol. 2011;89:62–69. [DOI] [PubMed] [Google Scholar]
- 10. Skurnik D, Merighi M, Grout M, et al. Animal and human antibodies to distinct Staphylococcus aureus antigens mutually neutralize opsonic killing and protection in mice. J Clin Invest. 2010;120:3220–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacDermott RP, Nash GS, Auer IO, et al. Alterations in serum immunoglobulin G subclasses in patients with ulcerative colitis and Crohn’s disease. Gastroenterology. 1989;96:764–768. [PubMed] [Google Scholar]
- 12. Scott MG, Nahm MH, Macke K, Nash GS, Bertovich MJ, MacDermott RP. Spontaneous secretion of IgG subclasses by intestinal mononuclear cells: differences between ulcerative colitis, Crohn’s disease, and controls. Clin Exp Immunol. 1986;66:209–215. [PMC free article] [PubMed] [Google Scholar]
- 13. M’Koma AE, Lindquist K, Liljeqvist L. A study of plasma immunoglobulins profile in connection with restorative proctocolectomy. Int J Surg Investig. 2000;2:125–131. [PubMed] [Google Scholar]
- 14. Ozawa T, Ishihara S, Tanaka T, et al. Clinical characteristics and postoperative complications of patients undergoing emergency surgery for ulcerative colitis. Hepatogastroenterology. 2015;62:853–858. [PubMed] [Google Scholar]
- 15. Bor R, Farkas K, Balint A, et al. Prospective comparison of magnetic resonance imaging, transrectal and transperineal sonography, and surgical findings in complicated perianal Crohn disease. J Ultrasound Med. 2016;35:2367–2372. [DOI] [PubMed] [Google Scholar]
- 16. Tsujikawa T, Andoh A, Sakaki M, et al. Operative indications for patients with refractory or severe ulcerative colitis. Hepatogastroenterology. 2005;52:1470–1473. [PubMed] [Google Scholar]
- 17. Um JW, M’Koma AE. Pouch-related dysplasia and adenocarcinoma following restorative proctocolectomy for ulcerative colitis. Tech Coloproctol. 2011;15:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zogg CK, Najjar P, Diaz AJ, et al. Rethinking priorities: cost of complications after elective colectomy. Ann Surg. 2016;264:312–322. [DOI] [PubMed] [Google Scholar]
- 19. Veress B, Reinholt FP, Lindquist K, Liljeqvist L. Different types of mucosal adaptation in the ileal reservoir after restorative proctocolectomy. A two-year follow-up study. APMIS. 1990;98:786–796. [DOI] [PubMed] [Google Scholar]
- 20. Veress B, Reinholt FP, Lindquist K, Lofberg R, Liljeqvist L. Long-term histomorphological surveillance of the pelvic ileal pouch: dysplasia develops in a subgroup of patients. Gastroenterology. 1995;109:1090–1097. [DOI] [PubMed] [Google Scholar]
- 21. Brandberg A, Kock NG, Philipson B. Bacterial flora in intraabdominal ileostomy reservoir. A study of 23 patients provided with “continent ileostomy.” Gastroenterology. 1972;63:413–416. [PubMed] [Google Scholar]
- 22. Philipson B, Brandberg A, Jagenburg R, Kock NG, Lager I, Ahren C. Mucosal morphology, bacteriology, and absorption in intra-abdominal ileostomy reservoir. Scand J Gastroenterol. 1975;10:145–153. [PubMed] [Google Scholar]
- 23. Loeschke K, Bolkert T, Kiefhaber P, et al. Bacterial overgrowth in ileal reservoirs (Koch pouch): extended functional studies. Hepatogastroenterology. 1980;27:310–316. [PubMed] [Google Scholar]
- 24. M’Koma AE. Serum biochemical evaluation of patients with functional pouches ten to 20 years after restorative proctocolectomy. Int J Colorectal Dis. 2006;21:711–720. [DOI] [PubMed] [Google Scholar]
- 25. Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6:e012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015:351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Puri KS, Suresh KR, Gogtay NJ, Thatte UM. Declaration of Helsinki, 2008: implications for stakeholders in research. J Postgrad Med. 2009;55:131–134. [DOI] [PubMed] [Google Scholar]
- 28. M’Koma AE, Wise PE, Muldoon RL, Schwartz DA, Washington MK, Herline AJ. Evolution of the restorative proctocolectomy and its effects on gastrointestinal hormones. Int J Colorectal Dis. 2007;22:1143–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ismaiel NE, Sharaf WM, Helmy DO, Zaki MM, Badawi MA, Soliman AS. Detection of cancer stem cells in colorectal cancer: histopathological and immunohistochemical study. Open Access Maced J Med Sci. 2016;4:543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dembe AE, Partridge JS, Geist LC. Statistical software applications used in health services research: analysis of published studies in the U.S. BMC Health Serv Res. 2011;11:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hommel G, Bretz F, Maurer W. Multiple hypotheses testing based on ordered p values—a historical survey with applications to medical research. J Biopharm Stat. 2011;21:595–609. [DOI] [PubMed] [Google Scholar]
- 32. Bretz F, Maurer W, Hommel G. Test and power considerations for multiple endpoint analyses using sequentially rejective graphical procedures. Stat Med. 2011;30:1489–1501. [DOI] [PubMed] [Google Scholar]
- 33. Shieh G. Estimation of the simple correlation coefficient. Behav Res Met. 2010;42:906–917. [DOI] [PubMed] [Google Scholar]
- 34. Pan Q, Hammarstrom L. Molecular basis of IgG subclass deficiency. Immunol Rev. 2000;178:99–110. [DOI] [PubMed] [Google Scholar]
- 35. Muller-Ladner U, Gross V, Andus T, et al. Distinct patterns of immunoglobulin classes and IgG subclasses of autoantibodies in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1996;8:579–584. [DOI] [PubMed] [Google Scholar]
- 36. Rai T, Wu X, Shen B. Frequency and risk factors of low immunoglobulin levels in patients with inflammatory bowel disease. Gastroenterol Rep (Oxf). 2015;3:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]