Abstract
IMPORTANCE
Cognitive behavior therapy (CBT) among youth with obsessive-compulsive disorder (OCD) is effective, but many patients remain symptomatic after intervention. D-cycloserine, a partial agonist at the N-methyl-D-aspartate receptor in the amygdala, has been associated with enhanced CBT outcome for OCD among adults but requires evaluation among youth.
OBJECTIVES
To examine the relative efficacy of weight-adjusted D-cycloserine (25 or 50 mg) vs placebo augmentation of CBT for youth with OCD and to assess if concomitant antidepressant medication moderated effects.
DESIGN, SETTING, AND PARTICIPANTS
In a placebo-controlled randomized clinical trial, 142 youths (age range, 7-17 years) enrolled between June 1, 2011, and January 30, 2015, at 2 academic health science centers (University of South Florida and Massachusetts General Hospital) with a primary diagnosis of OCD were randomized in a double-blind fashion to D-cycloserine plus CBT or placebo plus CBT. Intent-to-treat analysis was performed.
INTERVENTIONS
Patients were randomly assigned in a 1:1 ratio to either 10 sessions of D-cycloserine plus CBT or placebo plus CBT. D-cycloserine (25 or 50 mg) or placebo was taken 1 hour before sessions 4 through 10.
MAIN OUTCOMES AND MEASURES
Children’s Yale-Brown Obsessive Compulsive Scale at randomization, biweekly, midtreatment, and posttreatment. Secondary outcomes included the Clinical Global Impressions–Severity or Clinical Global Impressions–Improvement, remission status, Children’s Depression Rating Scale, Multidimensional Anxiety Scale for Children, and Children’s Obsessive-Compulsive Impact Scale–Parent Version.
RESULTS
The study cohort comprised 142 participants. Their mean (SD) age was 12.7 (2.9) years, and 53.5% (76 of 142) were female. A mixed-effects model using all available data indicated significant declines in the Children’s Yale-Brown Obsessive Compulsive Scale total score and Clinical Global Impressions–Severity. No significant interaction between treatment group and changes in the Children’s Yale-Brown Obsessive Compulsive Scale and Clinical Global Impressions–Severity indicated that the D-cycloserine plus CBT group and the placebo plus CBT group declined at similar rates per assessment point on the Children’s Yale-Brown Obsessive Compulsive Scale total score (estimate, −2.31, 95% CI, −2.79 to −1.83 and estimate, −2.03, 95% CI, −2.47 to −1.58, respectively) and Clinical Global Impressions–Severity (estimate, −0.29, 95% CI, −0.35 to −0.22 and estimate, −0.23, 95% CI, −0.29 to −0.17, respectively). No group differences in secondary outcomes were present. Antidepressant medication use at baseline did not moderate changes for either group.
CONCLUSIONS AND RELEVANCE
D-cycloserine augmentation of CBT did not confer additional benefit relative to placebo among youth with OCD. Other augmentation approaches should be examined to enhance outcome.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00864123
Pediatric obsessive-compulsive disorder (OCD) is a common,1 chronic, and disabling condition.2,3 Although most youths with OCD respond to cognitive behavior therapy (CBT) with exposure and response prevention (E/RP) or serotonin reuptake inhibitors,4,5 many patients remain symptomatic after receiving therapy. Pharmacological interventions are efficacious but rarely produce remission,6 may have adverse effects,7 and may not be an acceptable intervention to parents.8 Therefore, there is a need for empirically demonstrated, acceptable, and safe treatment augmentation approaches.
A novel pharmacological augmentation strategy is based on data suggesting that the N-methyl-D-aspartate receptor in the amygdala is critically involved in fear extinction and that the N-methyl-D-aspartate partial agonist D-cycloserine enhances extinction of learned fear.9 Exposure therapy, which is an efficacious treatment component of CBT for anxiety and OCD, partly relies on extinction learning.10 It is hypothesized that acutely dosed D-cycloserine can augment exposure therapy, particularly when the anxiety-provoking trigger has been successfully extinguished.11–13 Initial studies of D-cycloserine augmentation of exposure therapy in anxiety have yielded mixed results, with evidence supporting this approach for acrophobia,14 social phobia,15,16 posttraumatic stress,17 and panic disorder,18 while others have found limited benefit in treating social phobia,19 posttraumatic stress,20–22 and panic disorder.23
There are 7 preliminary randomized, double-blind, placebo-controlled trials of D-cycloserine vs placebo augmentation of CBT with E/RP in OCD, 3 within pediatric samples. Wilhelm et al24 randomized 23 adults to either placebo (n = 13) or 100 mg of D-cycloserine (n = 10) 1 hour before each of 10 E/RP sessions. Group differences emerged at midtreatment favoring the D-cycloserine arm (d = 1.17) but were not statistically significant posttreatment and at 1-month follow-up, although effects favoring D-cycloserine were large (d = 0.63 and d = 0.66).25 Kushner et al26 randomized 32 participants to either placebo (n = 17) or 125 mg of D-cycloserine (n = 15) 2 hours before each of 10 CBT sessions. Relative to the placebo arm, the D-cycloserine arm had significantly more rapid reductions in obsession-related fear ratings (d = 0.77) and required 2 fewer sessions than the placebo group to attain a greater than 50% reduction on all hierarchy items but did not differ in posttreatment Yale-Brown Obsessive Compulsive Scale scores. Storch et al27 randomized 24 adults with OCD to either placebo (n = 12) or 250 mg of D-cycloserine (n = 12) 4 hours before each of 12 CBT sessions. No significant group Yale-Brown Obsessive Compulsive Scale score differences existed posttreatment (d = −0.19) or in the rate of response. Andersson et al28 found no main effect of 50 mg of D-cycloserine relative to placebo augmentation (n = 128) of 12 internet CBT sessions. Concomitant serotonin reuptake inhibitor medication moderated effects such that among patients randomized to D-cycloserine augmentation those who were not taking a serotonin reuptake inhibitor were significantly more likely to achieve remission at follow-up (60% vs 24%). Serotonin reuptake inhibitor status was not associated with remission for the placebo augmentation arm.
Among children, Storch et al29 found small but non–statistically significant effects (d = 0.31) for weight-adjusted D-cycloserine vs placebo (25 or 50 mg) dosed 1 hour before 7 CBT sessions (n = 30). Farrell et al30 demonstrated a greater improvement in D-cycloserine–augmented relative to placebo-augmented CBT at 1-month follow-up on clinician-rated obsessional severity and diagnostic severity and on parent-rated OCD severity (n = 17). Mataix-Cols et al31 found that 50 mg of D-cycloserine or placebo administered immediately after 10 CBT sessions was not associated with differential efficacy (n = 27).
Taken together, there is support for the potential of D-cycloserine to amplify CBT response in OCD and its safety and acceptability in youth,32 which requires evaluation in a fully powered study. More recent evidence suggests that beneficial effects may be adversely moderated by concomitant antidepressant medication.28 Accordingly, we evaluated in a double-blind, placebo-controlled design whether D-cycloserine augments CBT efficacy to a greater extent than placebo. We hypothesized that D-cycloserine vs placebo augmentation would be associated with greater overall response and a faster rate of treatment response. We also examined if concomitant antidepressant use moderated treatment effects.
Methods
Participants
Two hundred six youths with a primary OCD diagnosis were enrolled between June 1, 2011, and January 30, 2015, at 2 sites (University of South Florida and Massachusetts General Hospital). Of these, 142 (age range, 7-17 years) were randomized to either D-cycloserine plus CBT or placebo plus CBT. Inclusion criteria were current and primary DSM-IV-TR OCD diagnosis established via clinical assessment and the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version,33 a score of at least 16 on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS),34 and at least 85 on the full-scale IQ.35 Children were excluded if any of the following criteria were met: (1) They initiated an antidepressant or antipsychotic medication within 12 or 6 weeks, respectively, before enrollment or had an increase in an established antidepressant dosage within 8 weeks before enrollment (6 weeks for antipsychotics). Medications were stable for 8 weeks before enrollment (6 weeks for anti-psychotics) and remained stable throughout treatment. (2) They had epilepsy, renal insufficiency, current or past substance abuse, generally poor physical health, weight less than 22.5 kg, or known D-cycloserine allergy. (3) They were unable to swallow study medication. (4) They had active suicidality or a suicide attempt in the past year. (5) They were pregnant or having unprotected sex (among female participants). (6) They had comorbid psychosis, bipolar disorder, autistic disorder, anorexia nervosa, or non-OCD primary hoarding symptoms.
Measures
Masked independent evaluators administered the measures and were trained through instructional meetings, in vivo observation, and ongoing direct supervision. Three video recordings of the CY-BOCS administered by the last author (D.A.G.) were used to train all raters to reliability to within 1 point of a criterion standard rating.
Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version
The Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version33 is a clinician-administered diagnostic interview for DSM-IV childhood disorders. It was administered at screening to both parents and children.
Children’s Yale-Brown Obsessive Compulsive Scale
The CY-BOCS34 is a psychometrically sound clinician-rated interview. It assesses OCD symptom severity.
Clinical Global Impressions–Severity and Clinical Global Impressions–Improvement
The Clinical Global Impressions–Severity (CGI-Severity) is a widely used 7-point clinician rating of severity (0 is no illness, and 6 is extremely severe symptoms).36 The Clinical Global Impressions–Improvement (CGI-Improvement) is a 7-point rating of treatment response (1 is very much improved, and 7 is very much worse). Scores of very much and much improved were used to define treatment responders.37,38
Child Obsessive-Compulsive Impact Scale–Parent
The Child Obsessive-Compulsive Impact Scale–Parent2 is a 56-item, parent-report measure. It assesses OCD-related impairment.
Multidimensional Anxiety Scale for Children
The Multidimensional Anxiety Scale for Children39 is a 39-item self-report questionnaire. It assesses anxiety symptoms.
Children’s Depression Rating Scale–Revised
The Children’s Depression Rating Scale–Revised40 is a semi-structured child interview. It assesses depression severity.
Procedures
The institutional review boards at the University of South Florida (USF) and Massachusetts General Hospital (MGH) approved the research procedures. Written parental informed consent and child assent were obtained. The full study protocol can be found in the Supplement. Thereafter, a trained masked independent evaluator with a master’s degree or higher administered the Schedule for Affective Disorders and Schizophrenia for School-Age Children– Present and Lifetime Version and the CY-BOCS to the parent and child together and then completed the CGI-Severity. Principal investigators (E.A.S. and D.A.G.) reviewed the diagnostic information at each site, and only individuals meeting criteria for a full DSM-IV OCD diagnosis were included. A pediatrician (D.A.G.) or child psychiatrist (T.K.M.) performed a baseline physical examination, and participants then completed the study measures. Laboratory values assayed included complete blood cell count, comprehensive metabolic panel, urine toxicology, and pregnancy test (for postpubescent female participants). Eligible participants began receiving CBT twice weekly for the initial 4 sessions. The CY-BOCS and CGI-Severity were administered before every second CBT session (study visits 5, 7, and 9) and before the seventh CBT session (visit 8). Participants who continued to meet eligibility criteria (ie, CY-BOCS score ≥16) were randomized before the fourth CBT session (study visit 5, which was the first session in which E/RP was conducted) by a computer-generated randomization program in a double-blind fashion to either 10 sessions of D-cycloserine plus CBT or placebo plus CBT (1:1 ratio). Within 1 week after the 10th CBT session, participants completed a postassessment, which included symptoms (ie, CY-BOCS score) and laboratory assays (complete blood cell count and metabolic panel) and a second physical examination. All clinical personnel (all authors except B.J.S.) and patients were masked to study arm assignment. Adverse events were monitored by the research team and an independent data and safety monitoring board. There were no serious adverse events.
Cognitive Behavior Therapy
Patients received 10 family-based CBT sessions over 8 weeks using an abbreviated treatment protocol.6 The initial 4 sessions were held twice weekly, and sessions 5 through 10 were weekly. Sessions 1 through 3 were devoted to psychoeducation, cognitive interventions, and hierarchy development. Sessions 4 through 10 were at least 5 days apart each and involved E/RP exercises specific to each youth in a graduated manner. Between-session homework was assigned (up to 1 hour daily) consisting of E/RP tasks similar to those addressed in sessions. Only sessions 4 through 10 were augmented with D-cycloserine or placebo. Master’s-level or doctorate-level clinical psychologists (S.S., A.H., J. Micco, J. McGuire, A.B.L., Chelsea Ale, and Marni Jacob) provided treatment under supervision. Treatment integrity was ensured through the use of session content checklists that corresponded to the treatment manual, weekly supervision, and evaluation of 20% of randomly selected audiotaped sessions.
D-Cycloserine
D-cycloserine and matching pill placebo were encapsulated by a site-specific investigational pharmacy using the same procedures into 25 mg with identical placebo capsules and, consistent with other research,12,15,24,28,29 were taken 1 hour before sessions 4 through 10 supervised by a research coordinator to ensure compliance. Two dosing levels were used, based on weight ranges, to ensure comparable levels (milligrams per kilogram). Children weighing 25 to 45 kg took 25 mg (approximately 0.56-1.0 mg/kg/d), and children weighing at least 46 kg took 50 mg provided in two 25-mg capsules (approximately 0.50-1.08 mg/kg/d).
Analytic Plan
We evaluated the sample on demographic characteristics and CY-BOCS scores at the point of randomization for differences as a function of treatment group or recruitment site, with χ2 or analysis of variance as appropriate. Analysis for the primary and secondary outcomes was conducted using linear mixed-effects models for continuous outcomes41,42 or generalized estimating equations for categorical outcomes, with treatment group (D-cycloserine plus CBT or placebo plus CBT) as the between-participant factor and time as the within-group factor, with α = .05. Analyses were also computed with a quadratic time component, but none of the interactions with treatment group were statistically significant and thus are not reported. The continuous outcome models used an unstructured covariance matrix and maximum likelihood estimation procedures. The present sample size was sufficient to detect a small-sized treatment group by linear time interaction (F = 0.09) and a medium-sized between-group difference (d = 0.47) at any single time point, with 0.80 power at α = .05. Follow-up analyses were conducted using antidepressant medication as a moderator of effect.
Results
Sample Characteristics
The Figure shows enrollment, randomization, and study completion status for the study arms. Two hundred six children (99 [48.1%] at USF and 107 [51.9%] at MGH) were recruited into the study. Of these children, 32 (16 [50.0%] at USF and 16 [50.0%] at MGH) did not meet the initial eligibility criteria, 24 (5 [20.8%] at USF and 19 [79.2%] at MGH) withdrew before randomization, and 8 (6 [75.0%] at USF and 2 [25.0%] at MGH) were enrolled but did not meet inclusion or exclusion criteria for randomization. Of the remaining 142 children, 72 (36 [50.0%] at USF and 36 [50.0%] at MGH) were randomized to the placebo plus CBT condition and 70 (36 [51.4%] at USF and 34 [48.6%] at MGH) to the D-cycloserine plus CBT condition. Three children in the D-cycloserine plus CBT condition dropped out before posttreatment. However, data from these participants were used in analyses, and the final analytic sample was 142 individuals.
Figure. Consolidated Standards of Reporting Trials Diagram.
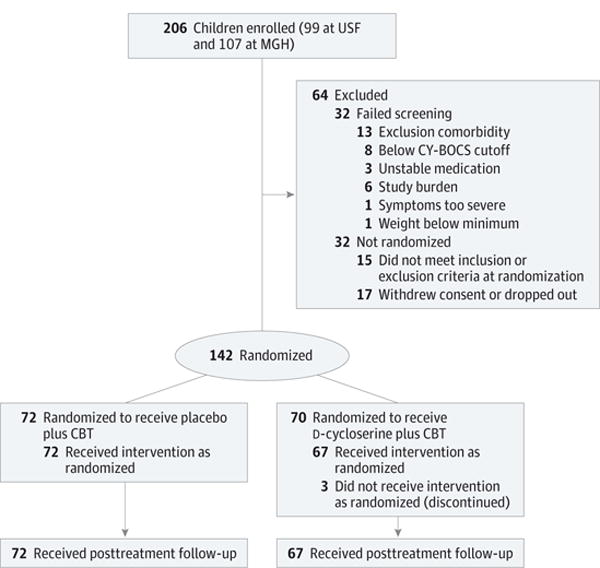
Details of study recruitment and retention are shown. CBT indicates cognitive behavior therapy; CY-BOCS, Children’s Yale-Brown Obsessive Compulsive Scale; MGH, Massachusetts General Hospital; and USF, University of South Florida.
Demographic characteristics by treatment group are listed in Table 1. There were no differences in child age as a function of treatment group (F1,138 = 1.02, P = .32), recruitment site (F1,138 = 0.31, P = .58), or group × site interaction (F1,138 = 0.00, P > .99). Child sex ( , P = .17) and race/ethnicity ( , P = .56) were comparable across treatment groups. There were no group differences in comorbid conditions (F1,138 = 0.03, P = .87), and the group × site interaction was not statistically significant (F1,138 = 0.00, P = .96). In terms of medication use, there were no group differences in the percentage of children taking antidepressant medication ( , = P = .44). Finally, there were no significant effects of treatment group (F1,138 = 0.77, P = .38) or the group × site interaction (F1,138 = 0.14, P = .71) for the CY-BOCS total score at randomization. However, there was a main effect of site on the CY-BOCS total score at randomization (F1,138 = 7.28, P = .008), with scores being higher at USF (mean [SD], 25.54 [6.01]) vs MGH (mean [SD], 23.11 [4.45]). Therefore, site was a covariate in the subsequent analyses.
Table 1.
Demographic Characteristics
| Variable | D-Cycloserine Plus CBT (n = 70) |
Placebo Plus CBT (n = 72) |
P Value |
|---|---|---|---|
| Age, mean (SE), y | 13.05 (2.93) | 12.54 (3.04) | .31a |
| Female sex, No. (%) | 42 (60.0) | 34 (47.2) | .13b |
| White race/ethnicity, No. (%) | 61 (87.1) | 65 (90.3) | .55b |
| CY-BOCS at randomization, mean (SE) | 24.74 (5.91) | 23.96 (4.91) | .39a |
| Overall comorbidities, No. (%) | |||
| OCD only | 11 (15.9) | 13 (18.6) | |
| OCD and 1 additional | 26 (37.7) | 15 (21.4) | .14c |
| OCD and 2 additional | 13 (18.8) | 22 (31.4) | |
| OCD and 3 or more | 19 (27.5) | 20 (28.6) | |
| Specific comorbid conditions, No. (%) | |||
| Generalized anxiety disorder | 18 (26.1) | 23 (31.4) | .49b |
| Socialphobia | 6 (23.2) | 9 (12.7) | .10b |
| Attention-deficit/hyperactivity disorder | 20 (29.4) | 17 (23.6) | .44b |
| Depressive disorder | 11 (15.9) | 10 (13.9) | .73b |
| Separation anxiety disorder | 9 (13.2) | 4 (5.6) | .12b |
| Psychotropic medication, % | |||
| Serotonin reuptake inhibitor | 17 (24.3) | 25 (34.7) | .17b |
| Atypicalantipsychotic | 0 | 3 (4.2) | .08b |
| Stimulant | 4(5.7) | 3 (4.2) | .67b |
Abbreviations: CBT, cognitive behavior therapy; CY-BOCS, Children’s Yale-Brown Obsessive Compulsive Scale; OCD, obsessive-compulsive disorder.
Analysis of variance F1,140.
Test.
Test.
Outcome Analyses
Table 2 lists the parameter estimates for the random-effects models for the primary outcomes of CY-BOCS total score and CGI-Severity, and Table 3 lists the secondary outcomes (Children’s Depression Rating Scale–Revised, Multidimensional Anxiety Scale for Children, and Child Obsessive-Compulsive Impact Scale–Parent). The key parameters of interest include the intercept (which reflects the mean performance at the point of randomization), time (which is the mean change per assessment for all participants), group (which indicates treatment group differences on the outcomes at the point of randomization), and the group × time interaction (which determines whether the treatment groups experience significant differences in longitudinal changes over treatment and at posttreatment). For the CY-BOCS scores and CGI-Severity, changes were indexed from the point of randomization (visit 5) to later points at visits 7, 8, and 9 and posttreatment. For the Children’s Depression Rating Scale–Revised, Multidimensional Anxiety Scale for Children, and Child Obsessive-Compulsive Impact Scale– Parent, changes were indexed from randomization (visit 5) to visit 8 and posttreatment. In addition to the fixed effects summarized in Table 2, a number of random effects are listed and index unexplained variation (residual), individual differences at the point of randomization (intercept), individual differences in longitudinal changes (slope), and the correlation between the intercept and the slope (correlation).
Table 2.
Parameter Estimates for Random-Effects Models on Outcomes
| Variable | β (SE) | ||||||
|---|---|---|---|---|---|---|---|
| CY-BOCS | CGI-Severity | CY-BOCS Compulsions | CY-BOCS Obsessions | CDRS | MASC | COIS-P | |
| Fixed effects | |||||||
| Intercept | 23.83 (0.93)a | 3.50 (0.13)a | 12.59 (0.49)a | 11.88 (0.51)a | 44.29 (1.69)a | 40.97 (3.64)a | 16.15 (1.61)a |
| Time | −2.03 (0.23)a | −0.23 (0.03)a | −1.08 (0.13)a | −1.00 (0.13)a | −1.15 (0.36)b | −1.63 (0.56)b | −1.43 (0.24)a |
| Site | −1.80 (1.31) | −0.14 (0.19) | −1.08 (0.69) | −1.37 (0.71) | 3.72 (2.47) | −2.88 (5.13) | −2.54 (2.28) |
| Group | 1.32 (1.31) | 0.25 (0.19) | 0.59 (0.69) | 0.27 (0.72) | −2.09 (2.39) | 7.27 (5.12) | −2.02 (2.27) |
| Group × site | 0.13 (1.86) | −0.04 (0.27) | 0.03 (0.97) | 0.55 (1.00) | 5.34 (3.59) | 1.13 (7.33) | 6.97 (3.24)c |
| Site × time | 0.29 (0.33) | −0.01 (0.04) | 0.15 (0.17) | 0.20 (0.18) | −0.33 (0.53) | 0.35 (0.80) | 0.56 (0.34) |
| Group × time | −0.28 (0.33) | −0.05 (0.04) | −0.14 (0.18) | −0.14 (0.18) | 1.02 (0.50)c | 0.23 (0.80) | 0.21 (0.34) |
| Group × site × time | 0.19 (0.47) | 0.03 (0.06) | 0.15 (0.24) | 0.03 (0.26) | 0.13 (0.78) | −0.35 (1.15) | −0.65 (0.49) |
| Random effects | |||||||
| Residual | 10.70 (0.74)a | 0.25 (0.02)a | 3.49 (0.25)a | 3.69 (0.26)a | 45.38 (5.95)a | 54.89 (7.28)a | 21.42 (2.77)a |
| Intercept | 25.16 (3.68)a | 0.50 (0.08)a | 6.18 (0.98)a | 6.62 (1.04)a | 67.27 (13.78)a | 416.77 (55.91)a | 74.65 (11.40)a |
| Slope | 1.23 (0.24)b | 0.02 (0.00)a | 0.29 (0.07)a | 0.33 (0.07)a | 0.81 (0.75) | 6.24 (1.47)a | 0.27 (0.33) |
| Correlation | −0.25 (0.11)a | −0.09 (0.13) | −0.21 (0.12) | −0.23 (0.12) | −0.16 (0.27) | −0.41 (0.10)a | −0.11 (0.27) |
Abbreviations: CDRS, Children’s Depression Rating Scale; CGI-Severity, Clinical Global Impressions-Severity; COIS-P, Children’s Obsessive-Compulsive Impact Scale-Parent Version; CY-BOCS, Children’s Yale-Brown Obsessive Compulsive Scale; MASC, Multi dimensional Anxiety Scale for Children.
P <.001.
P < .01.
P < .05.
Table 3.
Estimated Means for the Primary and Secondary Outcomes by Group
| Variable | Mean (95% CI) | Group Comparison | ||
|---|---|---|---|---|
| Placebo Plus CBT | D-Cycloserine Plus CBT | t Statistic | P Value | |
| CY-BOCS | ||||
| Randomization | 22.93 (21.11–24.74) | 24.33 (22.48–26.18) | 1.01 | .32 |
| Visit 7 | 21.05 (19.35–22.74) | 22.26 (20.54–23.98) | 0.85 | .40 |
| Visit 8 | 19.17 (17.47–20.86) | 20.19 (18.47–21.91) | 0.62 | .54 |
| Visit 9 | 17.29 (15.48–19.10) | 18.12 (16.27–19.97) | 0.36 | .72 |
| Posttreatment | 13.53 (11.22–15.85) | 13.98 (11.60–16.36) | −0.06 | .95 |
| CGI-Severity | ||||
| Randomization | 3.43 (3.17–3.69) | 3.67 (3.41–3.94) | 1.35 | .18 |
| Visit 7 | 3.19 (2.94–3.44) | 3.39 (3.14–3.65) | 1.12 | .26 |
| Visit 8 | 2.95 (2.69–3.20) | 3.12 (2.86–3.37) | 0.82 | .41 |
| Visit 9 | 2.71 (2.44–2.98) | 2.84 (2.56–3.11) | 0.50 | .62 |
| Posttreatment | 2.23 (1.89–2.56) | 2.28 (1.94–2.63) | −0.04 | .97 |
| CY-BOCS Compulsions | ||||
| Randomization | 12.05 (11.10–13.00) | 12.67 (11.71–13.62) | 0.85 | .40 |
| Visit 7 | 11.05 (10.16–11.93) | 11.59 (10.71–12.48) | 0.70 | .49 |
| Visit 8 | 10.04 (9.17–10.92) | 10.53 (9.65–11.41) | 0.49 | .63 |
| Visit 9 | 9.04 (8.10–9.98) | 9.46 (8.51–10.40) | 0.25 | .80 |
| Posttreatment | 7.03 (5.83–8.23) | 7.32 (6.09–8.54) | −0.13 | .90 |
| CY-BOCS Obsessions | ||||
| Randomization | 11.20 (10.22–12.19) | 11.76 (10.78–12.75) | 0.38 | .70 |
| Visit 7 | 10.29 (9.39–11.21) | 10.74 (9.86–11.64) | 0.20 | .84 |
| Visit 8 | 9.39 (8.49–10.30) | 9.71 (8.80–10.61) | −0.01 | .99 |
| Visit 9 | 8.49 (7.53–9.46) | 8.68 (7.70–9.66) | −0.20 | .84 |
| Posttreatment | 6.69 (5.45–7.94) | 6.63 (5.36–7.90) | −0.47 | .64 |
| CDRS | ||||
| Randomization | 46.15 (42.69–49.61) | 46.59 (42.96–50.23) | −0.88 | .38 |
| Visit 8 | 43.51 (40.47–46.56) | 46.13 (42.96–49.29) | −0.03 | .98 |
| Posttreatment | 39.56 (35.87–43.25) | 45.42 (41.61–49.24) | 1.16 | .25 |
| MASC | ||||
| Randomization | 39.53 (32.35–46.72) | 47.39 (40.07–54.72) | 1.42 | .16 |
| Visit 8 | 36.62 (30.13–43.11) | 44.59 (37.95–51.24) | 1.67 | .10 |
| Posttreatment | 32.24 (25.40–39.08) | 40.40 (33.33–47.47) | 1.73 | .09 |
| COIS-P | ||||
| Randomization | 14.88 (11.68–18.07) | 16.28 (13.05–19.50) | −0.89 | .38 |
| Visit 8 | 12.58 (9.58–15.58) | 13.74 (10.71–16.77) | −0.75 | .45 |
| Posttreatment | 9.14 (5.86–12.41) | 9.94 (6.64–13.24) | −0.42 | .67 |
Abbreviations: CBT, cognitive behavior therapy; CDRS, Children’s Depression Rating Scale; CGI-Severity, Clinical Global Impressions-Severity; COIS-P, Children’s Obsessive- Compulsive Impact Scale-Parent Version; CY-BOCS, Children’s Yale-Brown Obsessive Compulsive Scale; MASC, Multidimensional Anxiety Scale for Children.
For the CY-BOCS total score, the mean score at randomization was 23.82, and there was a significant effect of time, with scores decreasing by more than 2 points per assessment after randomization. The lack of a significant group × time interaction indicated that both the D-cycloserine plus CBT and placebo plus CBT groups experienced comparable changes over time (estimate, −2.31, 95% CI, −2.79 to −1.83 and estimate, −2.03, 95% CI, −2.47 to −1.58, respectively). A similar pattern of effects emerged with the CGI-Severity (estimate, −0.29, 95% CI, −0.35 to −0.22 and estimate, −0.23, 95% CI, −0.29 to −0.17, respectively). Antidepressant medication use did not moderate the group × time interaction for the CY-BOCS (β = 0.26, SE = 0.47; P = .59) or CGI-Severity (β = 0.03, SE = 0.07; P = .60). Finally, we examined differences in response and remission rates (CY-BOCS total scores ≤14 and ≤12) as a function of group (Table 4 lists group percentages). There were significant changes over time for CY-BOCS scores of at least 14 (β = 0.58, SE = 0.09; P < .001), CY-BOCS scores of at least 12 (β = 0.57, SE = 0.11; P < .001), and CGI-Improvement (β = 0.64, SE = 0.12; P < .001). However, there were no group × time interactions for CY-BOCS scores of at least 14 (β = 0.08, SE = 0.13; P = .58), CY-BOCS scores of at least 12 (β = 0.09, SE = 0.16; P = .56), and CGI-Improvement (β = 0.29, SE = 0.19; P = .14).
Table 4.
Percentage of Treatment Responders and Remitters as a Function of Group Statusa
| Variable | Responders, No. (%) | Group Comparison | ||
| Placebo Plus CBT | D-Cycloserine Plus CBT | Statistic | P Value | |
| CY-BOCS <14 | ||||
| Visit 7 | 9 (12.5) | 12 (17.1) | 0.61 | .44 |
| Visit 8 | 15 (20.8) | 12 (17.1) | 0.31 | .58 |
| Visit 9 | 27 (37.5) | 23 (32.9) | 0.34 | .56 |
| Posttreatment | 44 (61.1) | 42 (60.0) | 0.02 | .89 |
| CY-BOCS <12 | ||||
| Visit 7 | 4 (5.6) | 11 (15.7) | 3.88 | .05 |
| Visit 8 | 11 (15.3) | 1 (10.0) | 0.89 | .35 |
| Visit 9 | 15 (20.8) | 13 (18.6) | 0.11 | .74 |
| Posttreatment | 33 (45.8) | 35 (50.0) | 0.25 | .62 |
| CGI-Improvement | ||||
| Visit 8 | 38 (52.8) | 24 (34.3) | 4.93 | .03 |
| Posttreatment | 52 (72.2) | 58 (82.9) | 2.29 | .13 |
Abbreviations: CBT, cognitive behavior therapy; CGI-Improvement, Clinical Global Impressions- Improvement; CY-BOCS, Children’s Yale-Brown Obsessive Compulsive Scale.
Ratings of very much and much improved on the CGI-Improvement were used to classify treatment response status.
For the Children’s Depression Rating Scale–Revised, there was a main effect of time and a significant group × time interaction, indicating that the groups changed at different rates across the 3 time points. Follow-up analyses revealed that the placebo plus CBT group experienced statistically significant declines of approximately 1 point per assessment (β = −1.13, 95% CI, −1.89 to −0.40; P = .003), whereas the D-cycloserine plus CBT group did not exhibit significant changes over time (β = −0.14, 95% CI, −0.86 to 0.57; P = .69). For the Multidimensional Anxiety Scale for Children and Child Obsessive-Compulsive Impact Scale–Parent scores, there were significant main effects of time but no group × time interactions.
Discussion
Although efficacious,5 there is a clear need to improve CBT for pediatric OCD. Preliminary data among youth29,30 and adults24 with OCD, a recent fully powered study28 among adults with OCD not taking an antidepressant, and several studies12–16 of non-OCD anxiety highlight the potential for D-cycloserine to safely augment CBT. However, we found no evidence in the present study that D-cycloserine augmentation of CBT was more effective than CBT monotherapy or that concomitant antidepressant medication adversely moderated outcomes. Moreover, inconsistent with other research,18,25 there was no evidence that D-cycloserine augmentation yielded expedited treatment gains relative to placebo augmentation.
These nonsignificant outcomes may be understood in several ways. First, between-group differences may be overshadowed by the strong efficacy of protocol-driven, family-based CBT. Because both treatment groups exhibited a large response, there may be a ceiling effect for further and faster improvement with CBT, which has been demonstrated by others.19,27 Second, youth with OCD have a heterogeneous symptom presentation that can include both fear-based symptoms (ie, contamination, aggressive or taboo obsessions, checking, and forbidden thoughts) and non–fear-based symptoms (ie, ordering and symmetry). Because D-cycloserine enhances fear extinction, it may be that its augmentation properties only affected fear-based symptoms. Therefore, a more individualized symptom-level analysis may be needed to detect its benefit. It is also possible that D-cycloserine augments learning of not only fear extinction but also fear reconsolidation.11 Fear learning and reconsolidation within less successful exposure sessions may have been enhanced by D-cycloserine, thereby masking or neutralizing augmented gains made during other more successful E/RP tasks. Indeed, D-cycloserine augmentation after successful exposures has been associated with enhanced therapeutic outcomes,12,13 while some evidence suggests that D-cycloserine after less successful within-session distress habituation can actually result in fear reconsolidation and attenuated outcomes.21 Unfortunately, we were not able to test this hypothesis in our present design. Third, initial D-cycloserine studies had small sample sizes,24 and some researchers detected symptom change only through subjective assessments of distress.26 The possible benefit of D-cycloserine to reduce subjective distress assessments faster and to a greater degree than placebo may not directly translate to the standardized rating scales used in this trial. Indeed, a recent meta-analysis5 that used only standardized rating scales found no significant benefit of D-cycloserine relative to placebo across 20 randomized clinical trials. Fourth, there is evidence that chronic antidepressant treatment impairs the acquisition and extinction of fear.43,44 Because many youth in this study received prior selective serotonin uptake inhibitor (SSRI) treatment, prolonged SSRI exposure may have impaired fear acquisition and extinction learning. Conversely, other animal research suggests that SSRIs and other behaviors can enhance fear extinction.45,46
Our study had some limitations. We did not systematically measure distress ratings within each exposure session, which prohibits examining whether D-cycloserine enhances outcomes when within-session distress reduction is achieved, which others have found.12,13 Despite outreach efforts to enhance diversity, the sample was primarily of white race/ ethnicity.
Conclusions
In a well-powered study, we found no evidence that D-cycloserine augments CBT relative to placebo in youth with OCD. These findings carry 3 important implications. First, given the multiple factors that can influence fear extinction processes central to E/RP, further research should investigate moderators of enhanced or expedited treatment response to D-cycloserine– augmented E/RP. These examinations may elucidate the nuanced interaction between D-cycloserine use and fear extinc-tioninhumansandidentifyspecificcharacteristicsofyouthwith OCD who may benefit from this treatment approach. Second, because D-cycloserine does not universally enhance or expedite symptom reductions for youth with OCD, other safe and tolerable approaches to enhance fear extinction in E/RP should be explored. Third, the meaningful improvement demonstrated by an abbreviated family-based CBT course independent of D-cycloserine is consistent with extant reports6,47 and highlights the importance of CBT dissemination.
Supplementary Material
Key Points.
Question
Is weight-adjusted D-cycloserine (25 or 50 mg) vs placebo augmentation of cognitive behavior therapy among youth with obsessive-compulsive disorder efficacious, and does concomitant antidepressant medication moderate treatment effects?
Findings
In a placebo-controlled, randomized clinical trial that included 142 youth with obsessive-compulsive disorder, D-cycloserine augmentation of cognitive behavior therapy did not demonstrate statistically different outcomes from placebo augmentation of cognitive behavior therapy.
Meaning
D-cycloserine augmentation of cognitive behavior therapy did not confer additional benefit relative to placebo among youth with obsessive-compulsive disorder.
Acknowledgments
Funding/Support: This work was supported by grants 1R01MH093381 (Dr Storch) and 5R01MH093402 (Dr Geller) from the National Institute of Mental Health.
Role of Funder/Sponsor: The funding organization was not involved in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, and approval of the manuscript; or decision to submit the manuscript for publication.
Conflict of Interest Disclosures
Dr Storch reported receiving research support from the National Institutes of Health, Agency for Healthcare Research and Quality, International OCD Foundation, and All Children’s Hospital Research Foundation; reported receiving royalties from Elsevier Publications, Springer, American Psychological Association, John Wiley & Sons Inc, and Lawrence Erlbaum; reported being a consultant for Prophase Inc and Rijuin Hospital in China; reported serving on the speaker’s bureau and scientific advisory board for the International OCD Foundation; and reported receiving research support from the All Children’s Hospital Guild Endowed Chair. Dr Wilhelm reported receiving research support in the form of free medication and matching placebo from Forest Laboratories for a National Institute of Mental Health–funded clinical trial; reported being a presenter for the Massachusetts General Hospital Psychiatry Academy in educational programs supported through independent medical education grants from pharmaceutical companies; reported receiving royalties from Elsevier Publications, Guilford Publications, and New Harbinger Publications; reported receiving salary support from Novartis; reported receiving speaking honoraria from various academic institutions and foundations, including the International OCD Foundation and Tourette Association of America; and reported receiving payment from the Association for Behavioral and Cognitive Therapies for her role as associate editor for the Behavior Therapy journal, as well as from John Wiley & Sons Inc for her role as associate editor for the Depression and Anxiety journal. Dr Sprich reported receiving book royalties from Oxford Press and Springer. Dr Henin reported receiving book royalties from Oxford Press and reported receiving consulting fees from Clintara LLC, Prophase Inc, Otsuka, Alkermes, and Pfizer. Dr Micco reported receiving book royalties from New Harbinger Publications. Dr McGuire reported receiving grant funding from the Tourette Association of America and National Institutes of Health. Dr Lewin reported receiving research support from the All Children’s Hospital Research Foundation, Centers for Disease Control and Prevention, and International OCD Foundation; reported serving on the speaker’s bureau for the Tourette Association of America and International OCD Foundation; reported receiving travel support from the Tourette Association of America, American Psychological Association, Anxiety and Depression Association of America, National Institute of Mental Health, and Rogers Memorial Hospital; reported receiving consulting fees from Bracket and Prophase Inc; reported receiving book royalties from Springer; reported receiving honoraria from Oxford Press, Children’s Tumor Foundation, and University of Central Oklahoma; and reported being on the scientific and clinical advisory board for the International OCD Foundation and the board of directors for the Society for Clinical Child and Adolescent Psychology and American Board of Clinical Child and Adolescent Psychology. Dr Murphy reported receiving research funding from Auspex Pharmaceuticals, National Institute of Mental Health, Shire Pharmaceuticals, Pfizer, F. Hoffmann– La Roche Ltd, AstraZeneca Pharmaceuticals, Centers for Disease Control and Prevention, Massachusetts General Hospital, Sunovion Pharmaceuticals, Neurocrine Biosciences, PANDAS Network, and Psyadon Pharmaceuticals. Dr Geller reported receiving grant support from the National Institutes of Health and a book honorarium from the American Academy of Child and Adolescent Psychiatry; reported receiving speaking honoraria for Advanced Institute lectures from the American Academy of Child and Adolescent Psychiatry and Massachusetts General Hospital Psychiatry Academy in educational programs supported through independent medical education grants from pharmaceutical companies; and reported receiving lifetime funding support from the International OCD Foundation, Tourette Association of America, McIngvale Family Foundation, Eli Lilly, Pfizer, and GlaxoSmithKline. No other disclosures were reported.
Additional Contributions
The following individuals contributed to data collection and/or analysis or provided writing or editing assistance. Study Coordinators or Research Assistants: P. Jane Mutch, PhD, Allison Kennel, ARNP, and Nicole McBride, BS (University of South Florida) and Alyssa Faro, BA, Kesley Ramsay, BA, Ashley Brown, BA, Andrew Mittelman, BA, Abigail Stark, BA, Allison Cooperman, BA, and Angelina Gomez, BA (Massachusetts General Hospital). Therapists: Chelsea Ale, PhD, Marni Jacob, PhD, Joseph McGuire, PhD, and Adam B. Lewin, PhD, ABPP (University of South Florida) and Aude Henin, PhD, Susan Sprich, PhD, and Kathleen Trainor, PhD (Massachusetts General Hospital). Independent Evaluators: Monica Wu, MS, Robert Selles, MS, and Cary Jordan, PhD (University of South Florida) and Jamie Micco, PhD, Jennifer Park, PhD, Christine Cooper-Vince, PhD, Anne Chosak, PhD, and Noah Berman, PhD (Massachusetts General Hospital). Data Center: Brent Small, PhD (University of South Florida). Marco Grados, MD (The Johns Hopkins University), Gary Geffken, PhD (University of Florida Health), Rebecca Betensky, PhD (Harvard University), Barbara Coffey, MD (The Mount Sinai Hospital), and Martin Franklin, PhD (University of Pennsylvania), served on the data and safety monitoring board and received an honorarium for their consultation. Jennifer Britton, PhD (University of Miami), David Greenblatt, MD (Tufts University), and David Pauls, PhD (Massachusetts General Hospital), contributed to the study (without compensation). We thank the National Institute of Mental Health for supporting this study and the patients who participated.
Footnotes
Author Contributions: Drs Storch and Geller had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Storch, Wilhelm, Murphy, Geller.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Storch, Wilhelm, Henin, Small, McGuire, Lewin, Geller.
Critical revision of the manuscript for important intellectual content: Storch, Wilhelm, Sprich, Henin, Micco, Small, Mutch, Lewin, Murphy, Geller. Statistical analysis: Storch, Small.
Obtained funding: Storch, Wilhelm, Geller. Administrative, technical, or material support: Storch, Wilhelm, Sprich, Henin, Micco, McGuire, Mutch, Lewin, Murphy, Geller.
Study supervision: Storch, Wilhelm, Micco, Lewin, Murphy, Geller.
References
- 1.Zohar AH. The epidemiology of obsessive-compulsive disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 1999;8(3):445–460. [PubMed] [Google Scholar]
- 2.Piacentini J, Peris TS, Bergman RL, Chang S, Jaffer M. Functional impairment in childhood OCD: development and psychometrics properties of the Child Obsessive-Compulsive Impact Scale–Revised (COIS-R) J Clin Child Adolesc Psychol. 2007;36(4):645–653. doi: 10.1080/15374410701662790. [DOI] [PubMed] [Google Scholar]
- 3.Lack CW, Storch EA, Keeley ML, et al. Quality of life in children and adolescents with obsessive-compulsive disorder: base rates, parent-child agreement, and clinical correlates. Soc Psychiatry Psychiatr Epidemiol. 2009;44(11):935–942. doi: 10.1007/s00127-009-0013-9. [DOI] [PubMed] [Google Scholar]
- 4.Geller DA, Biederman J, Stewart SE, et al. Which SSRI? a meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder. Am J Psychiatry. 2003;160(11):1919–1928. doi: 10.1176/appi.ajp.160.11.1919. [DOI] [PubMed] [Google Scholar]
- 5.McGuire JF, Piacentini J, Lewin AB, Brennan EA, Murphy TK, Storch EA. A meta-analysis of cognitive behavior therapy and medication for child obsessive-compulsive disorder: moderators of treatment efficacy, response, and remission. Depress Anxiety. 2015;32(8):580–593. doi: 10.1002/da.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.March JS, Foa E, Gammon P, et al. Pediatric OCD Treatment Study (POTS) Team Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969–1976. doi: 10.1001/jama.292.16.1969. [DOI] [PubMed] [Google Scholar]
- 7.Murphy TK, Segarra A, Storch EA, Goodman WK. SSRI adverse events: how to monitor and manage. Int Rev Psychiatry. 2008;20(2):203–208. doi: 10.1080/09540260801889211. [DOI] [PubMed] [Google Scholar]
- 8.Stevens J, Wang W, Fan L, Edwards MC, Campo JV, Gardner W. Parental attitudes toward children’s use of antidepressants and psychotherapy. J Child Adolesc Psychopharmacol. 2009;19(3):289–296. doi: 10.1089/cap.2008.0129. [DOI] [PubMed] [Google Scholar]
- 9.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60(4):369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 10.Lewin AB, Wu MS, McGuire JF, Storch EA. Cognitive behavior therapy for obsessive-compulsive and related disorders. Psychiatr Clin North Am. 2014;37(3):415–445. doi: 10.1016/j.psc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann SG, Wu JQ, Boettcher H. D-cycloserine as an augmentation strategy for cognitive behavioral therapy of anxiety disorders. Biol Mood Anxiety Disord. 2013;3(1):11. doi: 10.1186/2045-5380-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smits JA, Rosenfield D, Otto MW, et al. D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J Psychiatr Res. 2013;47(10):1455–1461. doi: 10.1016/j.jpsychires.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smits JA, Rosenfield D, Otto MW, et al. D-cycloserine enhancement of fear extinction is specific to successful exposure sessions: evidence from the treatment of height phobia. Biol Psychiatry. 2013;73(11):1054–1058. doi: 10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann SG, Meuret AE, Smits JA, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63(3):298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 16.Guastella AJ, Richardson R, Lovibond PF, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63(6):544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Difede J, Cukor J, Wyka K, et al. D-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacology. 2014;39(5):1052–1058. doi: 10.1038/npp.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otto MW, Tolin DF, Simon NM, et al. Efficacy of D-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67(4):365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann SG, Smits JA, Rosenfield D, et al. D-cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. Am J Psychiatry. 2013;170(7):751–758. doi: 10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heresco-Levy U, Kremer I, Javitt DC, et al. Pilot-controlled trial of D-cycloserine for the treatment of post-traumatic stress disorder. Int J Neuropsychopharmacol. 2002;5(4):301–307. doi: 10.1017/S1461145702003061. [DOI] [PubMed] [Google Scholar]
- 21.Litz BT, Salters-Pedneault K, Steenkamp MM, et al. A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res. 2012;46(9):1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Scheeringa MS, Weems CF. Randomized placebo-controlled D-cycloserine with cognitive behavior therapy for pediatric posttraumatic stress. J Child Adolesc Psychopharmacol. 2014;24(2):69–77. doi: 10.1089/cap.2013.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegmund A, Golfels F, Finck C, et al. D-cycloserine does not improve but might slightly speed up the outcome of in-vivo exposure therapy in patients with severe agoraphobia and panic disorder in a randomized double blind clinical trial. J Psychiatr Res. 2011;45(8):1042–1047. doi: 10.1016/j.jpsychires.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm S, Buhlmann U, Tolin DF, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165(3):335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- 25.Chasson GS, Buhlmann U, Tolin DF, et al. Need for speed: evaluating slopes of OCD recovery in behavior therapy enhanced with D-cycloserine. Behav Res Ther. 2010;48(7):675–679. doi: 10.1016/j.brat.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Kushner MG, Kim SW, Donahue C, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62(8):835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Storch EA, Merlo LJ, Bengtson M, et al. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2007;22(4):230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- 28.Andersson E, Hedman E, Enander J, et al. D-cycloserine vs placebo as adjunct to cognitive behavioral therapy for obsessive-compulsive disorder and interaction with antidepressants: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):659–667. doi: 10.1001/jamapsychiatry.2015.0546. [DOI] [PubMed] [Google Scholar]
- 29.Storch EA, Murphy TK, Goodman WK, et al. A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010;68(11):1073–1076. doi: 10.1016/j.biopsych.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrell LJ, Waters AM, Boschen MJ, et al. Difficult-to-treat pediatric obsessive-compulsive disorder: feasibility and preliminary results of a randomized pilot trial of D-cycloserine–augmented behavior therapy. Depress Anxiety. 2013;30(8):723–731. doi: 10.1002/da.22132. [DOI] [PubMed] [Google Scholar]
- 31.Mataix-Cols D, Turner C, Monzani B, et al. Cognitive-behavioural therapy with post-session D-cycloserine augmentation for paediatric obsessive-compulsive disorder: pilot randomised controlled trial. Br J Psychiatry. 2014;204(1):77–78. doi: 10.1192/bjp.bp.113.126284. [DOI] [PubMed] [Google Scholar]
- 32.Byrne S, Farrell LJ, Storch EA, Rapee RM. D-cycloserine augmented treatment of anxiety disorders in children and adolescents: a review of preliminary research. Psychopathol Rev. 2014;1(1):157–168. [Google Scholar]
- 33.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 35.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corp; 1999. [Google Scholar]
- 36.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Dept of Health, Education, and Welfare, Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976. Clinical Global Impressions scale. [Google Scholar]
- 37.Storch EA, Lewin AB, De Nadai AS, Murphy TK. Defining treatment response and remission in obsessive-compulsive disorder: a signal detection analysis of the Children’s Yale-Brown Obsessive Compulsive Scale. J Am Acad Child Adolesc Psychiatry. 2010;49(7):708–717. doi: 10.1016/j.jaac.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Farris SG, McLean CP, Van Meter PE, Simpson HB, Foa EB. Treatment response, symptom remission, and wellness in obsessive-compulsive disorder. J Clin Psychiatry. 2013;74(7):685–690. doi: 10.4088/JCP.12m07789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Poznanski EO, Mokros HB. Children’s Depression Rating Scale, Revised (CDRS-R) Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- 41.Hoffman L. Longitudinal Analysis: Modeling Within-Person Fluctuation and Change. New York, NY: Routledge; 2015. [Google Scholar]
- 42.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. Cary, NC: SAS Institute Inc; 2006. p. 814. [Google Scholar]
- 43.Burghardt NS, Sigurdsson T, Gorman JM, McEwen BS, LeDoux JE. Chronic antidepressant treatment impairs the acquisition of fear extinction. Biol Psychiatry. 2013;73(11):1078–1086. doi: 10.1016/j.biopsych.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55(12):1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Riddle MC, McKenna MC, Yoon YJ, et al. Caloric restriction enhances fear extinction learning in mice. Neuropsychopharmacology. 2013;38(6):930–937. doi: 10.1038/npp.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karpova NN, Pickenhagen A, Lindholm J, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334(6063):1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franklin ME, Sapyta J, Freeman JB, et al. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: the Pediatric OCD Treatment Study II (POTS II) randomized controlled trial. JAMA. 2011;306(11):1224–1232. doi: 10.1001/jama.2011.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


